Abstract
In a rabbit model of collagenase-induced osteoarthritis, the additive effects of intra-articular recombinant human growth hormone (GH) administration to hyaluronic acid (HA) were evaluated. After intra-articular collagenase injection, mature New Zealand white rabbits (n=30) were divided into 3 groups. Group 1 (control rabbits) received once weekly intra-articular saline injections for 4 weeks. Group 2 rabbits received 6 mg HA injections, and group 3 rabbits were injected with 6 mg HA and 3 mg recombinant human GH. These injections were initiated 4 weeks after collagenase injections. Lameness was observed for 9 weeks after collagenase injections. Macroscopic and histopathological knee joint findings were also evaluated at the end of 9 weeks after collagenase injections. Although all animals had lameness after collagenase injections, the duration and severity of lameness were significantly shorter and less severe in group 3 than group 1 and 2 (P<0.01). Macroscopic scores showed that femoral condyles of group 3 rabbits received significantly less cartilage damage than those of groups 1 and 2 rabbits (P<0.01). Histopathological score was also the lowest in group 3 (P<0.01). These results suggest that co-injection of intra-articular HA and recombinant human GH is more effective than HA injections alone in an osteoarthritis model.
Osteoarthritis (OA) is a degenerative joint disease characterized by progressive articular cartilage loss, subchondral bone sclerosis, osteophyte formation, synovial membrane changes, and an increased synovial fluid with decreased viscosity and lubrication properties. Mechanical, biochemical, and genetic factors are all involved in OA pathogenesis (1).
Non-surgical modalities for OA management include weight loss, exercise, activity modification, assistive devices, non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, and intra-articular glucocorticoid and hyaluronic acid (HA) preparations (2). NSAIDs and glucocorticoids are the most commonly used treatments among these modalities. However, due to the adverse effects on cartilage and other potential side effects, the role of NSAIDs in OA management is controversial (3). Cortisone, strong anti-inflammatory agent, is also limited in use by articular cartilage damage, secondary infection, and systemic side effects with repeated injections (4).
HA has been recognized as a safe and efficacious alternative therapy. It binds to the proteoglycan surface components of articular cartilage, provides an important boundary layer for lubrication, prevents cartilage destruction, influences anti-inflammatory properties, and facilitates cartilage re-arrangement. The efficacy of HA therapy is dependent on OA grade. While moderate to mild OA is responsive to HA, it is not effective in cases with considerable effusion or in individuals with gross architectural changes (7).
Growth hormone is an important regulator of skeletal growth and bone mineral density. It also stimulates cartilage growth probably through local and systemic IGF-1 production and possibly by direct stimulation of cartilage cell proliferation. Circulating GH or one of its mediators may accelerate osteochondral defect healing by stimulating osseous and chondral tissue formation (8). Morphoangiogenesis was initially identified in the knees of adult rabbits undergoing intra-articular GH injections for articulophyseal cartilage regeneration (9). In this experiment, the regeneration cascade resulted in the transformation of central arteries in the subchondral osteones into tortuous, thin-walled fenestrated capillary structures which contained erythrocytes, histiocytes, stem cells, and chondrocytes. This morphoangiogenesis might promote generative and constructive action in joints. The exact mechanisms of intra-articular GH are unclear, but synovial fluid GH could enhance the proliferation, matrix synthesis, and differentiation of bone and cartilage cells in vitro (4). In this study, we evaluated the effects of intra-articular HA and recombinant human GH administration of collagenase-induced OA in rabbits.
Male New Zealand white rabbits (n=30) aged 12 weeks were used in the experiments. Animals were housed in separate metal cages at a temperature of 23±2℃ and a relative humidity of 45±10%. The animals were allowed free access to tap water and were fed a commercial rabbit diet. Animal experiments were performed in accordance with internationally accredited guidelines, and were approved by each laboratory's Institutional Animal Care and Use Committee.
Experimental animals were anesthetized with an intramuscular xylazine injection at a dose of 1.9 mg/kg (Rompun, Bayer Co., Seoul, Korea) and 46 mg/kg of ketamine (Ketar, Yuhan Co., Seoul, Korea). The right knee joints of all rabbits were shaved and sterilized, and 2 mg collagenase type II from Clostridium histolyticum (Sigma Co., St. Louis, MO, USA) was prepared for intra-articular injection. Collagenase was dissolved in sterile phosphate buffered saline (pH 7.4) and filtered with a 0.22 µm membrane. The solution was slowly injected into the right knee joint cavity. The same collagenase injection procedure was performed 3 days after the first injection, as previously described (10).
Rabbits were divided into 3 groups of 10 rabbits apiece. The control group (group 1) received once weekly intra-articular injections of saline (0.6 mL) for 4 weeks. Injections were given 4 weeks after collagenase injections. Group 2 rabbits received intra-articular HA (Hyruan-plus®, LG Life Science, Daejeon, Korea) injections (6 mg). The HA molecular weight measured at 3.0×106 Dalton, and it was prepared to a 10 mg/mL concentration. Group 3 rabbits received intra-articular HA (6 mg) and recombinant human GH (3 mg) injections. All procedures were performed under general anesthesia (intraperitoneal xylazine and ketamine) and sterile conditions. No post-operative medications were administered. All animals were euthanized by intravenous injection (sodium pentobarbital, 100 mg/kg) 9 weeks after initial collagenase injection.
Clinical observations were performed once daily, on 14:00 in a day. The animals were turned out to the ground of the area of 2 square meters, then gait was assessed by direct observation for 20 min individually. In the intact limb, knee and ankle angles go through a typical flexion and extension cycle during hoping. Lameness was defined as a non-weightbearing of the affected limb and a losing of typical flexion and extension cycle during hoping in comparison with contralateral side. The severity of lameness was not quantified. The times to normal ambulation without non-weigthbearing lameness of affected limb were recorded, and lameness periods were calculated on each group. Three independent physiatrists without prior knowledge of the experimental groups performed the observation.
Knee joints were dissected after euthanasia. The medial and lateral condyles of the femur and tibia were examined for gross changes. Cartilage surfaces of the loaded areas of the femur and tibia were evaluated for degeneration, using the scoring system by Yoshimi et al. (11). All measurements were performed on the lateral part of the femoral condyle, which in the region most affected by collagenase (10). Normal cartilage was scored as 0, softened cartilage as 1, fibrillation as 2, erosion as 3, ulceration as 4, and loss of cartilage as 5.
The lateral and medial condyles of the femur and tibia were fixed with 10% neutral buffered formalin and decalcified with 20% EDTA. Calcified condyles were embedded in paraffin, and standard frontal sections of 5 µm microsections were prepared and stained with hematoxylin and eosin in the lateral part of the femoral condyle cartilage according to gross observation (10). If the stain is not proper, we cut the specimen next cartilage surface. Cartilage degradation features were analyzed using the scoring system modified by Mankin et al. (12). Histopathological evidence of cartilage degeneration was evaluated by structural scoring (0, normal; 1, surface irregularities; 2, pannus and surface irregularities; 3, clefts to transitional zones; 4, clefts to radial zones; 5, clefts to calcified zones; and 6, complete disorganization) and cell status (0, normal; 1, diffuse hypercellularity; 2, cloning; and 3, hypocellularity) of the articular cartilage. Total scores ranged from 0 (normal) to 9 (complete disorganization and hypocellularity of articular cartilage). All sections were graded by two independent observers that were kept unaware of the treatment groups.
Side effects including injection site infection, swelling, anorexia, diarrhea, and vomiting were not observed during 9 weeks evaluation period. Time to recovery of normal ambulation was an average of 26 days in group 1 rabbits, 19.1 days in group 2 rabbits, and 11.6 days in group 3 rabbits (Table 1).
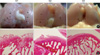
Macroscopic damages in the femoral condyles of treated rabbits differ with treatment regime: saline, HA, coinjection of HA and GH (Fig. 1). All the evaluated group 3 rabbits showed smooth surfaces and integration of repair tissues to the surrounding articular cartilage in the femoral condyles, suggesting healing without degenerative effects. On the contrary, loss of cartilage and erosion were observed in the condyles of group 1 and 2 rabbits. Macroscopic scores in control and treatment groups are shown in Table 1. HA injected or HA and GH coinjected groups showed significant lower macroscopic scores compared with the control group. Consistent with macroscopic features, group 3 rabbits, coinjected with GH with HA, was also lower in macroscopic score than group 2 injected with HA alone (Table 1).
The control group (group 1 rabbits) demonstrated complete disorganization of articular cartilage with apparent cloning of chondrocytes in the transitional and radial zones. Group 2 rabbits demonstrated clefts extending to the transitional zone with normal cellularity in the transitional and radial zones. Group 3 rabbits had normal cartilage surfaces with normal cellularity in the transitional and radial zones (Fig. 1). Histopathological scores were 1.4 in group 3 rabbits, 4.0 in group 2 rabbits, and 6.9 in group 1 rabbits. Group 3 scores were significantly lower than group 1 and 2 scores (P<0.01), as seen in Table 1.
The objective of this study was to evaluate the additive effect of recombinant human GH to intra-articular HA treatment. Unlike other joint conditions, OA is local disease restricted to one or more joints. This unique feature provides an opportunity for local intra-articular treatment without the risk of systemic side effects (13).
Articular cartilage and synovial fluid play an essential role in the movement of joints. Synovial fluid provides an effective hydrodynamic lubricant by forming a thin viscous film of low shear rates over the surface of the synovium and articular cartilage in the joint space. Articular cartilage spreads compressive stresses over the articular plate surfaces of the joint, thus protecting weight-bearing bones from shattering or deteriorating. The cartilage matrix of joint is maintained by a balance between the synthesis by chondrocyte and their subsequent degradation by proteolytic enzymes from chondrocytes. Chronic disruption of this equilibrium is associated with the development of osteoarthritis (14, 15). HA contributes to the viscoelastic property of synovial fluid that serves as a lubricant and a shock absorber (16). It also plays an important role in the maintenance and restoration of cartilage matrix through inhibiting the formation of prostaglandins, inducing proteoglycan aggregation, and modulating the inflammatory response (4-6). In OA, both the concentration and molecular weight of intra-articular endogenous HA are decreased (6). Intra-articular HA injection is widely recognized as an effective treatment of OA. The efficacy of intra-articular HA injection can be influenced by molecular weight of injected HA (16, 17). Molecules with higher molecular weights may yield more superior clinical results than those with lower molecular weights in OA treatment (11, 18). Considering these molecular weight effects on efficacy, high molecular weight HA (3.0×106 Dalton) was used in the present study.
In the present study, injection of HA alone yielded better clinical outcomes with less macroscopic and histopathologic damage than the control group (Fig. 1, Table 1). However, intra-articular HA injection did not fully restore the cartilage defect in collagenase-induced OA (Fig. 1, Table 1). HA injected rabbits (group 2), demonstrated macroscopic erosions in the knee cartilage and clefts extending to the transitional zone in the histopathologic evaluation. There is previous report that intra-articular HA injections were not effective in cases with considerable effusion or in individuals with gross architectural changes (7).
Combined therapy with HA and recombinant human GH showed better results than HA alone in clinical observations and macroscopic and histopathological findings. Articular cartilage was completely healed and revealed normal surfaces and cellularity in group 3 rabbits (Fig. 1, Table 1). In this study, GH was given in a dose of 3 mg that is comparable to dose used in GH deficit patients or previous reports about the GH in the tibial fracture of rabbits (14).
Until recently, the effect of exogenous GH administration in the process of skeletal repair remains controversial. Bak et al. (14) studied 36 rats with closed diaphyseal tibial fractures with intramedullary nailing. In this study, all rats received subcutaneous recombinant human GH injections (1 mg) twice per day. Significant improvement in maximal stiffness and ultimate load-bearing were observed at evaluation performed after 40 days of healing. Kolbeck et al. (19) reported that the administration of homologous GH stimulates callus formation and ossification in the early phase of bone healing, which consequently results in an increased mechanical strength and stiffness. On the contrary, GH administration had no measurable effects on fracture healing in a standardized tibia osteotomy rabbit model (20).
Kolbeck et al. (19) studied 23 dogs with experimentally-induced 3 cm ulnar bone defects and intramedullary fixation. Dogs were injected with recombinant bovine GH (1 mg), and several of these GH-treated dogs had closure of these bone defect while the remainder demonstrated healing.
New form of angiogenesis, morphoangiogenesis, was reported in the knee of adult rabbits with intra-articular GH injections for articulophyseal cartilage regeneration (9). Instead of four layered daughter vessels identical to their parent vessels in regular angiogenesis, capillaries with single layer fenestrated thin wall were formed by intra-articular growth hormone induced morphoangiogenesis. Dunn (9) claimed that this architecture is suitable for the production of stem cells and regeneration of articulophyseal cartilage. In present study, recombinant human GH was injected into intra-articular spaces using the method similar to Dunn's report (9), suggesting the possible involvement of morphoangiogenesis in the additive effects of GH to intra-articular HA injections.
Short residence time due to rapid uptake by circulation imposes major challenge in intra-articular delivery of recombinant human GH (13). This emphasizes the need for the development of sustained-release formulas that support the continuous release of the drug from a depot in the joint space over several weeks to months.
Previously-marketed daily injections have provided distinct peaks and troughs of GH concentrations over 24 hr. The pharmacokinetic profile of recombinant GH is different from that of normal physiologic GH with distinct bursts (21). Genetech and Alkermes developed the Nutropin Depot® as a sustainedrelease human GH formulation using polylactic glycolic acid (PLGA) microparticles for the first time. Nutropin Depot® seemed to achieve stable therapeutic IGF-1 target levels for at least 14 days with higher efficacy and without supraphysiological GH concentrations at all times. Nutropin Depot® required the fewest injection number to achieve GH levels within the target range, and appealed to patient in its convenience and compliance. However, the PLGA particle size of microspheres was too large to be suspended efficiently in an injection medium. Therefore, injections through a 21 to 23 gauge are inevitable. Complexities with long-acting protein delivery system using PLGA include inflammation and protein denaturation by hydrophobic interactions and harsh acidic microenvironments. These complexities decrease the bioavailability of Nutropin Depot® (22).
The recombinant human GH used in the present study is a sustained-release formula which induced continuous serum IGF-1 elevation for 6 days after a single injection. This product also exhibited greater than 95% bioavailability. Recombinant human GH in medium chain triglycerides can be easily injected through a 26 to 27 gauge needle due to the small particle size and the localized lecithin on the microparticle surfaces. However, considering that this study was conducted in a relatively short period to assess long-term effects, additional studies must be performed to assess the differences in intra-articular dosage, formulations, and injection method variability.
In conclusion, our results suggest that co-injection of intra-articular HA and recombinant human GH is more effective than HA injections alone in an osteoarthritis model. Novel combined therapy of HA with chondrocyte protective functions and recombinant human GH with generative and constructive actions in OA affected joint can be promising treatment option for OA.
Figures and Tables
Fig. 1
(A) Gross findings of the femoral condyles in Group 1 (G1), Group 2 (G2) and Group 3 (G3) rabbits. (B) histopathologic findings of axial sections obtained at the rectangular areas shown in column A photographs (H&E staining, ×40). The loss of cartilage is seen on the femoral condyles (black arrows).
ACKNOWLEDGEMENTS
The authors appreciate the efforts of Inbum Chung and Mi Sung Kim in preparation of the manuscript.
References
1. Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev. 2006. 7:239–250.

2. Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995. 38:1541–1546.
3. Behrens F, Shepard N, Mitchell N. Alterations of rabbit articular cartilage by intra-articular injection of glucocorticoids. J Bone Joint Surg Am. 1975. 57:70–76.
4. Goldspink DF, Goldberg AL. Influence of pituitary growth hormone on DNA synthesis in rat tissues. Am J Physiol. 1975. 228:302–309.

5. Frizziero L, Govoni E, Bacchini P. Intra-articular hyaluronic acid in the treatment of osteoarthritis of the knee: clinical and morphological study. Clin Exp Rheumatol. 1998. 16:441–449.
6. Adams ME, Atkinson MH, Lussier AJ, Schulz JI, Siminovitch KA, Wade JP, Zummer M. The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthritis Cartilage. 1995. 3:213–225.

7. Namiki O, Toyoshima H, Morisaki N. Therapeutic effect of intraarticular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int J Clin Pharmacol Ther Toxicol. 1982. 20:501–507.
8. Bail H, Klein P, Kolbeck S, Krummrey G, Weiler A, Schmidmaier G, Haas NP, Raschke MJ. Systemic application of growth hormone enhances the early healing phase of osteochondral defects-a preliminary study in micropigs. Bone. 2003. 32:457–467.

10. Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage. 1998. 6:177–186.

11. Yoshimi T, Kikuchi T, Obara T, Yamaguchi T, Sakakibara Y, Itoh H, Iwata H, Miura T. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop Relat Res. 1994. 298:296–304.

12. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971. 53:523–537.
13. Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006. 58:226–242.

14. Bak B, Jørgensen PH, Andreassen TT. Increased mechanical strength of healing rat tibial fractures treated with biosynthetic human growth hormone. Bone. 1990. 11:233–239.

15. Coutts RD, Sah RL, Amiel D. Effects of growth factors on cartilage repair. Instr Course Lect. 1997. 46:487–494.
16. Mihara M, Higo S, Uchiyama Y, Tanabe K, Saito K. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthritis Cartilage. 2007. 15:543–549.

17. Kobayashi Y, Okamoto A, Nishinari K. Viscoelasticity of hyaluronic acid with different molecular weights. Biorheology. 1994. 31:235–244.

18. Brockmeier SF, Shaffer BS. Viscosupplementation therapy for osteoarthritis. Sports Med Arthrosc. 2006. 14:155–162.

19. Kolbeck S, Bail H, Schmidmaier G, Alquiza M, Raun K, Kappelgard A, Flyvbjerg A, Haas N, Raschke M. Homologous growth hormone accelerates bone healing-a biomechanical and histological study. Bone. 2003. 33:628–637.

20. Carpenter JE, Hipp JA, Gerhart TN, Rudman CG, Hayes WC, Trippel SB. Failure of growth hormone to alter the biomechanics of fracture-healing in a rabbit model. J Bone Joint Surg Am. 1992. 74:359–367.

21. Laursen T, Jørgensen JO, Jakobsen G, Hansen BL, Christiansen JS. Continuous infusion versus daily injections of growth hormone (GH) for 4 weeks in GH-deficient patients. J Clin Endocrinol Metab. 1995. 80:2410–2418.

22. Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly (lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000. 17:100–106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download