Abstract
Bleeding into joint space is critical to develop hemophilic arthropathy. To reduce the frequency of bleeding in the ankle joint of children with hemophilic arthropathy, low dose external beam irradiation was performed for 37 patients. Among them, 35 patients followed-up for longer than 1 yr (median 87 months) were enrolled for analysis. The average number of bleedings per month was 3.6 during one year prior to radiation therapy. After radiation therapy, it was decreased to 2.1 during the first year, after then it was maintained in the range of 1.0 to 1.5 until the tenth year. The bleeding frequency was reduced to 42% at the first year and it was maintained in the range of 58% to 73% from the second to the tenth year. Especially the patients who had 3 or more bleedings per month, and who had MRI score more than 3 showed significant decreases. During the follow-up period, growth disturbances and secondary malignancies were not found. External beam radiotherapy can be considered for the hemophilic patients with surgical or isotope therapies are not amenable.
In patients with hemophilia, 85% of all bleeding events occur in joints, and the joint-related morbidity remains the largest source of disability (1). Furthermore, the incidence of target joints, which is defined as to have 3 or more bleeds within 3 months, increases with age approaching up to 20% by age 18 yr (2). The blood in the joint space initiates hypertrophy of the synovium, release of proteolytic enzymes, and hemosiderin deposits. Contractures can also occur, leading to the end-stage of hemophilic arthropathy (3). After repetitive bleeding, the hemarthrosis results in chronic synovitis, epiphysial overgrowth, and the destruction of cartilage. The repetitive joint bleeding eventually leads to a vicious cycle of hemarthrosis-synovitis-hemarthrosis (4). Thus, managing the number of instances of bleeding into the joint space is critical for prevention of the vicious cycle of hemophilic arthropathy.
Currently, arthroscopic or radioisotopic synovectomies with or without factor infusion are the mainstay of treatments for hemophilic arthropathy. However, for patients with inhibitory antibodies, multiple affected joints, tight regulation of factor replacement, limited resources, or in circumstances where rehabilitation cannot be ensured, treatment options more feasible than those mentioned above are warranted.
In the meantime, patients with hemophilic pseudotumor, which is defined as progressive cystic swellings caused by recurrent hemorrhage, treated with external beam radiotherapy (EBRT) have been reported to have complete remission even with very low administered doses (5, 6). In our institution, a considerable number of hemophilic pseudotumor cases showed decreased bleeding frequencies in the irradiated joints. Thus, even though the mechanism of reduced joint bleeding after EBRT is not well understood, EBRT was attempted to reduce bleeding frequencies for hemophilic patients with repetitive bleeding in ankle joints that were not amenable to arthroscopic or radioisotopic synovectomies.
In the previous report with hemophilic arthropathy patients treated with external beam radiotherapy with one year follow-up, the bleeding frequency was reduced in thirty-three cases among forty-one joints (7) and the patients aged younger than 11 yr (n=23) showed better results than 11 yr or older patients (n=18). However, the previous report included variable joints (35 ankle joints, 3 knee joints and 3 elbow joints) with variable ages (ranged from 4 to 27 yr) and the follow-up period was too short (one year) so that it was not sufficient to evaluate the effect of EBRT on bleeding frequency of the hemophilic arthropathy patients.
Therefore a long term follow up results of EBRT for hemophilic arthropathy were retrospectively analyzed for the examination of bleeding frequencies and related morbidities such as bone growth or secondary malignancies.
From 1997 to 2006, a total of 37 hemophilic patients under age 18 yr were treated with external beam radiation therapy. Among them, 35 patients were followed-up for longer than one year. The number of patients includes 24 patients who were enrolled for previous report (7). This retrospective study was approved by the Institutional Review Board at the Kyung Hee Medical Center (KMC IRB 1014-02).
Detailed patient data, including age at the time of radiotherapy, type of hemophilia, involved joint, initial radiographic joint stage by plain radiography according to the Arnold-Hilgartner's (A-H) staging system (8), joint scores by MRI according to the suggestion of Funk et al. (9), and pretreatment bleeding frequency were reviewed retrospectively.
All patients were assessed by pediatric hemophilia specialists, an orthopedic surgeon, and a radiologist to decide treatment options. All children with moderate or severe factor-deficiency were eligible for this protocol if they had moderate to severe changes in a target joint as manifested on a physical examination and plain radiographs of the joints. A target joint was defined as a joint in which clinically evident bleeding, as assessed by patient, parent and/or provider, had occurred 3 or more times in a 3-month period (2). However, all of the patients enrolled to external beam radiation therapy were classified to have target joints, so we further defined the patients with joint bleeding 3 or more times per month to have a "critical target joint". These patients were considered to have a higher probability of not respond to conventional therapy and may develop profound disabilities.
External beam irradiation was performed with a 6 MV radiography by Clinac 2100C (Varian Co., Palo Alto, CA, USA). The upper margin of the irradiated field was 2 cm above the epiphyseal plate of the distal tibia and the lower margin was 3 cm below the distal fibular tip to encompass the entire joint space (Fig. 1A). The fraction size and total dose were decided based on the patient's age and epiphyseal status. The radiation doses for patients younger than 11 yr were limited to no more than 9 Gy in order to prevent possible bony growth disturbance. Dose fractions used were 1.5 Gy for 28 patients, 2 Gy for 6 patients, and 3 Gy for 1 patient. Total doses administered were 9 Gy for 28 patients, 12 Gy for 2 patients, 18 Gy for 1 patient, 20 Gy for 3 patients, and 30 Gy for 1 patient. An 18-yr-old patient with severe pain and a pseudotumor in the distal tibia was treated with 30 Gy in 3-Gy fractions.
The patients were followed regularly to monitor their bleeding and any morbidities related to the treatment. At every visit, the treated joint was evaluated clinically for range of motion, presence of pain, and the frequency of bleeding during the period. Also, the patients were followed to monitor any effect on bone growth and any development of neoplasia. Follow-up radiological studies were not done routinely, unless clinically indicated.
Student t-test, Kaplan-Meier analysis, and Cox regression analysis were used for data analysis. P value <0.05 was considered statistically significant.
The number of patients treated at the left, right, and at both ankle joints were 12, 13, and 10, respectively. Ten patients had inhibitors for the hemophilic factor. The median age was 9.1 yr, ranging from 4 to 18 yr. The Arnold-Hilgatner's stage III was 15, while IV was 20. MRI evaluation before radiotherapy was performed in 26 patients, in whom there were 12 affected joints with MRI scores higher than 3, and 14 with scores of 3 or less (Table 1).
The average number of bleedings per month during the 1 yr period prior to radiation therapy was 3.6. After radiation therapy, the frequency was decreased to 2.1 during the first year, after which time it was maintained in the range of 1.0 to 1.5 until the tenth year (Fig. 2). The bleeding frequency was reduced to 42% at the first year and maintained in the range of 58% to 73% from the second to the tenth year.
The patients were classified into two groups according to the number of bleedings during the 1 yr period prior to EBRT. As mentioned above, patients with 3 or more bleedings per month were considered to have a critical target joint. Before EBRT, 18 patients were defined to have a critical target joint and 17 patients were not. In patient group with critical target joint, the average number of bleedings per month during the 1 yr period prior to radiation therapy was 5.0. After radiation therapy, the average number of bleedings was decreased to 2.7 during the first year, after which time it was maintained in the range of 1.3 to 2.0 until the tenth year. In patient group without critical target joint, the average number of bleedings per month during the 1 yr period prior to radiation therapy was 2.0. After radiation therapy, the average number of bleedings was decreased to 1.5 during the first year, after which time the number of bleedings was maintained in the range of 0.6 to 1.5 until the tenth year (Fig. 3). The patients with critical target joint showed a statistically significant reduction in bleeding frequency compared to the patients without critical target joint (P=0.018) (Fig. 4). However, some of the patients initially did not have critical target joint were found to develop critical target joint after radiation therapy. In these patients, the critical target joint was found in 11.7% for the first year and it was found until fourth year after radiation therapy. In the patients with critical target joint, those percent was 33.3% for the first year and range of 0% to 33.3% until eighth year (Fig. 5).
The patients were also classified into two groups according to their Arnold-Hilgatner's stage (stage III vs IV). Fifteen joints were in Arnold-Hilgatner's stage III, and 20 joints were in stage IV. Post-EBRT average bleeding frequencies were compared between the two groups. The joints of the group with Arnold-Hilgatner's stage IV showed a statistically significant decreased bleeding frequency compared to patients with stage III (P=0.004) (Fig. 6).
Magnetic resonance imaging evaluation before radiotherapy was performed in 26 patients, and the patients were classified into two groups according to MRI score (≤3 vs >3). There were 14 patients with an MRI score of 3 or less, and 12 with an MRI score greater than 3. The patients with an MRI score of higher than 3 showed a significantly reduced bleeding frequency compared to patients with lower MRI scores (P=0.012) (Fig. 7).
The patients were further classified according to age (19 patients younger than 10 yr vs 16 patients over 10 yr old), and radiation dose (27 patients receiving less than 10 Gy vs 8 patients receiving 10 Gy or more). There was no statistically significant difference according to age (P=0.331) or radiation dose (P=0.447). On multivariate analysis, only pre-EBRT bleeding frequency (P=0.029) and MRI score (P=0.025) showed statistically significant relationship with decreased bleeding frequency (Table 2).
During the follow-up period, growth disturbances and secondary malignancies were not found in any of the patients.
The primary management of hemophilic hemarthrosis is directed to stop bleeding with immediate factor replacement. To control recurrent bleeding, it is necessary to maintain prophylactic factor replacement, but prophylactic clotting factor infusion has limitations. In contrast to the widespread availability of clotting factor in the western world, the supply of clotting factor is not sufficient in some countries. It is estimated that approximately 75% of hemophilic patients do not receive proper treatment (10) partly because of the high cost of these products (11). Furthermore, after the development of a target joint, mean factor VIII usage needs to be increased by approximately 116% (12). Furthermore, presence of antibodies against the factor may hamper the prevention of joint bleedings. With these reasons, the usefulness of factor replacement in the control of repetitive joint bleeding is more or less limited.
The use of arthroscopic synovectomy to control joint bleeding showed an approximately 70% reduction in instances of bleeding with markedly less frequent loss of joint motion than open synovectomy (13). However, in patients with advanced arthropathy and fibrosis, extensive debridement is required to reduce the chance that the arthroscopic synovectomy could become complicated by significant hemorrhage in the perioperative period (14). Furthermore, the procedure may require higher doses of clotting factor and hospitalization (15). In addition, this procedure is not easily performed on children because small joints such as ankle joints are less accessible to arthroscopic procedures. Young children may also be less able to cooperate with postoperative physical therapy after arthroscopic synovectomy, which could result in a limited range of motion for the joint (14). The removal of synovium may reduce the rate of progression of damage, but the joint damage intrinsic to the cartilage will continue to proceed in a pediatric patient (10).
For nonsurgical management, radioisotope (RI) synovectomy is currently in use with remarkable results. In pediatric patients, Mathew et al. (16) reported that they observed marked improvement in 13 of 17 procedures and indicated that the procedure was feasible, safe, and efficacious in the treatment of hemophilic arthropathy in pediatric patients. However, the potential complications of RI synovectomy include leakage of the isotope and accumulation of small radioactive particles in the surrounding lymph nodes and reticuloendothelial system (17). Also, joint immobilization after the procedure is required to minimize leakage (18). As with the concerns relating to factor replacement, a large portion of hemophilic patients worldwide do not have access to RI synovectomy, either due to limited availability or due to its high costs (19).
Thus for patients with inhibitory antibodies, multiple affected joints, tight regulation of factor replacement, limited resources, or in situations where rehabilitation cannot be ensured, a more feasible treatment option than surgical arthroscopy or RI synovectomy is needed.
In the current report, the patients showed considerable reduction in bleeding frequency, so that the number of bleeding per month was reduced to 42% of the number of before EBRT at the first year and maintained in the range of 58% to 73% from the second to the tenth year. The results are slightly better than previous report where the number of bleeding per month was decreased to 55% of the number of before EBRT (from 2.52 to 1.4). However, previous report (7) included variable joints (35 ankle joint, 3 knee joint and 3 elbow joints) with variable ages (ranged from 4 to 27 yr). The difference may come from that the only patients under age 18 yr were enrolled to the current analysis. In the previous report, we reported that there was a tendency of frequent bleeding for the patients younger than 11 yr. Thus it is suggested that the response to radiation is better in younger patients.
Pre-EBRT bleeding frequency, Arnold-Hilgatner's stage, and MRI score were found to be prognostic factors affecting post-EBRT bleeding frequency reduction by univariate analysis, while only pre-EBRT bleeding frequency and MRI score were identified as prognostic factors affecting post-EBRT bleeding frequency reduction by multivariate analysis. The average post-EBRT bleeding frequency reduction rate was slightly lower than the reported reduction rates of radioisotope synovectomy and arthroscopic synovectomy. However, the authors did not find any EBRT-associated complications such as loss of joint motion, transient flu-like symptoms, localized joint pain, joint swelling, infection, or periprocedure hemarthrosis, and EBRT does not necessitate hospitalization or joint immobilization and requires no coverage with clotting factor.
In addition, possible bony growth disturbances, which may be induced by radiation, are another major concern. In children, growing epiphyses are particularly radiosensitive. Radiation-induced bony growth disturbances apparently result from an arrest in chondrogenesis and vascular changes. The effects of radiation on bone growth are more serious for higher doses of radiation and for younger patients. Silverman et al. (20) reported that the risk of epiphyseal slippage is about 47% in children irradiated while under the age of 4 yr, and about 5% in older children. The frequency of occurrence rose dramatically for those patients who were less than 4 yr old at time of irradiation. However, in the relationship between dose and age at irradiation, no slippage occurred if less than 25 Gy were received by the epiphyseal plate. In conclusion, they found that patients below the age of 4 yr who received doses in excess of 25 Gy to the epiphyseal plate were at particular risk for slippage, and that it is the dose of radiation to the epiphysis that is the major cause of the complication. Hogeboom et al. (21) reported that with the external beam radiation therapy in 2,778 children with Wilms tumor, they observed height deficits was zero for 16 children who received RT doses under 15 Gy at a mean age of 83 months. In our study, the authors delivered radiation doses for patients younger than 11 yr old of no more than 9 Gy. And, during the follow-up period, bony growth disturbances were not seen.
In summary, EBRT decreases intraarticular bleeding frequency in hemophilic arthritis patients with minimal complications. However, similar to radioisotope synovectomy, radiation-induced chromosomal changes and secondary malignancies are a major concern with EBRT, and therefore, long-term studies are needed.
Figures and Tables
Fig. 1
Image of treated ankle joint. (A) The simulation film for the radiation therapy. (B) Coronal T1-weighted magnetic resonance image showing prominent areas of hemosiderin, hyperplastic synovium adjacent to the ankle joint, some erosion, and small amount of effusion to give the score 6.
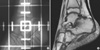
Fig. 2
The average number of bleedings for each year. Before radiation therapy, the number of bleeding was 3.6 per month. After radiation therapy, the average number of bleedings was decreased to 2.1 for the first year, and then the number was maintained in the range of 1.0 to 1.5 until the tenth year.

Fig. 3
The average number of bleeding for each year in patient group with pre-EBRT bleeding frequency 3 or more and pre-EBRT bleeding frequency less than 3. After radiation therapy, the average number of bleeding was maintained in the range of 1.3 to 2.7 in patient group with pre-EBRT bleeding frequency 3 or more, 0.6 to 1.5 in patient group with pre-EBRT bleeding frequency less than 3, respectively.

Fig. 4
The joints of group with pre-EBRT bleeding frequency 3 or more per month showed statistically significant decreased bleeding frequency (P=0.018). The patient was defined to have sustained bleeding when the bleeding frequency did not show statistically significant improvement in relation with pretreatment bleeding frequency by T-test.

Fig. 5
The percent of patients having critical target joint was in the range of 0% to 11.7% from the first to the tenth year in patient group with pre-EBRT bleeding frequency less than 3 per month. In patient group with pre-EBRT bleeding frequency 3 or more, the range was 0% to 33.3%. Patients with bleeding frequency less than 3 per month were well maintained without critical target joints from 5 yr after radiation therapy.

Fig. 6
Joints of the group with Arnold-Hilgatner's stage IV show statistically significant decreased bleeding frequency (P=0.004).

References
1. Roosendaal G, Lafeber FP. Blood-induced joint damage in hemophilia. Semin Thromb Hemost. 2003. 29:37–42.

2. Dunn AL. Management and prevention of recurrent hemarthrosis in patients with hemophilia. Curr Opin Hematol. 2005. 12:390–394.

3. Hansen ES, Hjortdal VE, Noer I, Christensen SB, Holm IE, Bunger C. 99mTc-DPD uptake in juvenile hemarthrosis. Scintimetry and autoradiography of the knee in dogs. Orthopedics. 1989. 12:441–447.
4. Raffini L, Manno C. Modern management of haemophilic arthropathy. Br J Haematol. 2007. 136:777–787.

5. Kang JO, Cho YJ, Yoo MC, Hong SE. Hemophilic pseudotumor of the ulna treated with low dose radiation therapy: a case report. J Korean Med Sci. 2000. 15:601–603.

6. Subasi M, Dirier A, Kapukaya A, Uludag A, Karadayi B, Cebesoy O. Successful treatment of hemophilic hand pseudotumors by only radiotherapy. Ann Plast Surg. 2007. 59:338–340.

7. Kang JO, Hong SE, Kim SG, Shin DO. The Effect of Radiation Therapy on Hemophilic Arthropathy. J Korean Soc Ther Radiol Oncol. 2005. 23:106–110.
8. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977. 59:287–305.

9. Funk MB, Schmidt H, Becker S, Escuriola C, Klarmann D, Klingebiel T, Kreuz W. Modified magnetic resonance imaging score compared with orthopaedic and radiological scores for the evaluation of haemophilic arthropathy. Haemophilia. 2002. 8:98–103.

10. Jansen NW, Roosendaal G, Lafeber FP. Understanding haemophilic arthropathy: an exploration of current open issues. Br J Haematol. 2008. 143:632–640.

11. Roosendaal G, Lafeber F. Prophylactic treatment for prevention of joint disease in hemophilia--cost versus benefit. N Engl J Med. 2007. 357:603–605.
12. Kern M, Blanchette V, Stain AM, Einarson TR, Feldman BM. Clinical and cost implications of target joints in Canadian boys with severe hemophilia A. J Pediatr. 2004. 145:628–634.

13. Wiedel JD. Arthroscopic synovectomy of the knee in hemophilia: 10-to-15 year followup. Clin Orthop Relat Res. 1996. (328):46–53.
14. Luck JV Jr, Silva M, Rodriguez-Merchan EC, Ghalambor N, Zahiri CA, Finn RS. Hemophilic arthropathy. J Am Acad Orthop Surg. 2004. 12:234–245.

15. Chang TJ, Mohamed S, Hambleton J. Hemophilic arthropathy: considerations in management. J Am Podiatr Med Assoc. 2001. 91:406–414.

16. Mathew P, Talbut DC, Frogameni A, Singer D, Chrissos M, Khuder S, Ohler S, Farley D, Michael C, Robinson MG. Isotopic synovectomy with P-32 in paediatric patients with haemophilia. Haemophilia. 2000. 6:547–555.

17. Erken EH. Radiocolloids in the management of hemophilic arthropathy in children and adolescents. Clin Orthop Relat Res. 1991. (264):129–135.

18. Gratz S, Gobel D, Behr TM, Herrmann A, Becker W. Correlation between radiation dose, synovial thickness, and efficacy of radiosynoviorthesis. J Rheumatol. 1999. 26:1242–1249.
19. Siegel ME, Siegel HJ, Luck JV Jr. Radiosynovectomy's clinical applications and cost effectiveness: a review. Semin Nucl Med. 1997. 27:364–371.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download