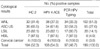
1. Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995. 87:796–802.

2. Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002. 55:244–265.

3. Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003. 88:63–73.

4. Munoz N, Bosch FX, de Sanjose S, Tafur L, Izarzugaza I, Gili M, Viladiu P, Navarro C, Martos C, Ascunce N. The causal link between human papillomavirus and invasive cervical cancer: a population-based case-control study in Colombia and Spain. Int J Cancer. 1992. 52:743–749.

5. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999. 189:12–19.

6. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007. 121:621–632.

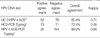
7. Castle PE, Lorincz AT, Mielzynska-Lohnas I, Scott DR, Glass AG, Sherman ME, Schussler JE, Schiffman M. Results of human papillomavirus DNA testing with the Hybrid Capture 2 assay are reproducible. J Clin Microbiol. 2002. 40:1088–1090.

8. Vince A, Kutela N, Iscic-Bes J, Harni V, Ivanisevic M, Sonicki Z, Culig Z, Poljak M. Clinical of molecular detection of human papillomavirus in cervical samples by hybrid capture technology. J Clin Virol. 2002. 25:Suppl 3. S109–S112.
9. Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005. 97:1066–1071.

10. de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995. 76:1057–1062.

11. Jacobs MV, Snijders PJ, van den Brule AJ, Helmerhorst TJ, Meijer CJ, Walboomers JM. A general primer GP5+/GP6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol. 1997. 35:791–795.

12. Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998. 36:3020–3027.
13. Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, Schiffman MH, Scott DR, Apple RJ. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000. 38:357–361.
14. Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, ter Harmsel B, Quint W. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998. 153:1731–1739.

15. Perrons C, Kleter B, Jelley R, Jalal H, Quint W, Tedder R. Detection and genotyping of human papillomavirus DNA by SPF10 and MY09/11 primers in cervical cells taken from women attending a colposcopy clinic. J Med Virol. 2002. 67:246–252.

16. Perrons C, Jelley R, Kleter B, Quint W, Brink N. Detection of persistent high-risk human papillomavirus infections with hybrid capture II and SPF10/LiPA. J Clin Virol. 2005. 32:278–285.
17. Takubo K, Shimomura-Izumiyama N, Koiwai H, Honma N, Esaki Y, Yoshida T, Nakajima T, Sawabe M, Arai T. Detection of human papillomavirus infection of the cervix in very elderly women using PCR. Clin Cancer Res. 2005. 11:2919–2923.

18. Fujinaga Y, Shimada M, Okazawa K, Fukushima M, Kato I, Fujinaga K. Simultaneous detection and typing of genital human papillomavirus DNA using the polymerase chain reaction. J Gen Virol. 1991. 72:1039–1044.

19. Worda C, Huber A, Hudelist G, Schatten C, Leipold H, Czerwenka K, Eppel W. Prevalence of cervical and intrauterine human papillomavirus infection in the third trimester in asymptomatic women. J Soc Gynecol Investig. 2005. 12:440–444.
20. Grayson W, Rhemtula HA, Taylor LF, Allard U, Tiltman AJ. Detection of human papillomavirus in large cell neuroendocrine carcinoma of the uterine cervix: a study of 12 cases. J Clin Pathol. 2002. 55:108–114.

21. Sandri MT, Lentati P, Benini E, Dell'Orto P, Zorzino L, Carozzi FM, Maisonneuve P, Passerini R, Salvatici M, Casadio C, Boveri S, Sideri M. Comparison of the Digene HC2 assay and the Roche AMPLICOR Human papillomavirus (HPV) test for detection of high-risk HPV genotypes in cervical samples. J Clin Microbiol. 2006. 44:2141–2146.

22. Stevens MP, Garland SM, Rudland E, Tan J, Quinn MA, Tabrizi SN. Comparison of the Digene Hybrid Capture 2 assay and Roche AMPLICOR and LINEAR ARRAY Human Papillomavirus (HPV) tests in detecting high-risk HPV genotypes in specimens from women with previous abnormal Pap smear results. J Clin Microbiol. 2007. 45:2130–2137.

23. Nazarenko I, Kobayashi L, Giles J, Fishman C, Chen G, Lorincz A. A novel method of HPV genotyping using Hybrid Capture sample preparation method combined with GP5+/6+ PCR and multiplex detection on Luminex XMAP. J Virol Methods. 2008. 154:76–81.

24. Castle PE, Porras C, Quint WG, Rodriguez AC, Schiffman M, Gravitt PE, Gonzalez P, Katki HA, Silva S, Freer E, van Doorn LJ, Jimenez S, Herrero R. Hildesheim A for the CVT group. Comparison of two PCR-based human papillomavirus genotyping methods. J Clin Microbiol. 2008. 46:3437–3445.
25. Chun JY, Kim JK, Hwang IT, Kim YJ, Lee DH, Lee IK, Kim JK. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007. 35:e40.

26. Denis F, Hanz S, Alain S. Clearance, persistence and recurrence of HPV infection. Gynecol Obstet Fertil. 2008. 36:430–440.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download