Abstract
Ethanol and its metabolite acetaldehyde increase transforming growth factor beta1 (TGF-β1) expression in animal studies. TGF-β1 is related with the hepatic stellate cell (the key element of hepatic fibrogenesis) and the radial glia (the key element of neuronal migration). Blood samples were collected from 41 patients with alcohol dependence, TGF-β1 levels measured by ELISA were compared with 41 normal subjects. Plasma TGF-β1 levels in the patients with alcohol dependence (1,653.11±532.45 pg/mL) were significantly higher than those of healthy subjects (669.87±366.53 pg/mL) (P=0.000). Patients with or without liver pathology showed no difference in TGF-β1 (P=0.36). Increased TGF-β1 may mediate deleterious effect of alcohol such as hepatic fibrosis and suppressed neuronal developments in alcohol dependence patients.
Chronic alcohol consumption is the most prevalent etiologic factor of hepatic fibrogenesis and cirrhosis (1). Ethanol is metabolized primarily by the alcohol dehydrogenase and the cytochrome P450 systems (2). Acetaldehyde generated by these oxidative pathways is accumulated due to slow kinetics of its oxidizing enzyme aldehyde dehydrogenase (3). Ethanol and acetaldehyde affect liver with directly generating reactive oxygen species (ROS) and indirectly producing transforming growth factor beta (TGF-β) (3). Patients with alcohol induced liver cirrhosis had significantly more leucine homozygous genotype polymorphism of TGF-β1 at codon 10 in Korean (4). TGF-β is a growth factor whose function is promotion of cell differentiation, fibrosis, angiogenesis, apoptosis, and suppression of cell proliferation (5, 6). TGF-β also mediates alcoholic liver diseases by hepatic apoptosis, hepatic fibrogenesis and suppression of hepatocyte proliferation (7, 8). Among three of its subtypes TGF-β1 is the most abundant and universally expressed isoform (9). In animal study, ethanol metabolite acetaldehyde increase autocrine TGF-β1 expression in hepatic stellate cells (10). This TGF-β1 increases the production of extracellular matrix proteins and decreases the matrixdegrading proteolytic enzymes (11). In addition to these liver fibrosis inductions, TGF-β damages alcoholic liver with triggering hepatocyte apoptosis, and inhibiting hepatocyte proliferation (7, 8). In nervous system, TGF-β1 controls neuronal migration and neurite outgrowth by regulating cell adhesion proteins (12, 13).
There were many reports of increased TGF-β in ethanol treated rats in previous animal studies (14, 15). But human alcoholism study comparable has not been. In order to verify the relation between alcohol dependence and TGF-β, we measured TGF-β in patients with alcohol dependence, and compared it with control subjects.
We assessed the peripheral TGF-β1 levels in the plasma of alcohol dependence patients and healthy controls. Forty-one male patients who met the Diagnostic and Statistical Manual of Mental Disorder IV (DSM-IV) criteria for alcohol dependence were interviewed and sampled. They were all admitted to psychiatric closed ward in Hangang Sacred Heart Hospital. Two or more psychiatrists at the hospital carried out the diagnosis in a consensus procedure using all available clinical material, including a semi-structured interview based on DSM-IV. Patients were excluded from the study if they had a history of comorbid psychiatric illness, addiction to drugs other than alcohol or nicotine, physical disorders other than alcohol-related medical illness, and any neurological disease. Medical consultation were done to find any liver pathology which could affect TGF-β1 level. The comparison subjects comprised 41 unrelated healthy males who were employees of Hangang Sacred Heart Hospital. Most of the participating employees were nondrinkers; only some were occasional light drinkers (persons who drink occasionally but have lower score than cut-off value of AUDIT-K), as revealed by a drinking-habit questionnaire. The study was approved by the hospital ethics committee (Han-Gang Sacred Heart Hospital).
Ten mL of venous blood was collected within 24 hr after admission and stored at -70℃. Plasma TGF-β1 level was determined by capture ELISA using monoclonal anti-human TGF-β1 antibody according to manufacturer's instructions (R&D Systems Inc., Minneapolis, MN, U.S.A.). In short, 100 µL of the capture antibody was transferred to ELISA plate and incubated overnight at room temperature. After washing wells with wash buffer, the plates were blocked by 300 µL of PBS containing 5% Tween 20, 5% sucrose and 0.05% NaN3 to each well and incubated at room temperature for 1 hr. Blood plasma was added 100 µL per each well. The ELISA plate was covered with an adhesive strip and incubated 2 hr at room temperature. Streptavidin HRP (R&D Systems, 1/200 in appropriate diluent) was added 100 µL per each well. Then the plate was covered and incubated for 20 min at room temperature. After subsequent addition of substrate solution and stop solution (R&D Systems), the optical density of each well was determined within 30 min at 450 nm.
Numerical values are expressed as mean±standard deviation. The Kolmogorov-Smirnov test showed that the TGF-β1 values did not fit a standard distribution curve, i.e. the distribution of the TGF-β1 levels were positively skewed. We used nonparametric Mann-Whitney test, Kruskal-Wallis test and correlation analysis with Statistica 7.0 (StatSoft Inc., Tulsa, OK, U.S.A.). The statistical significance level was set at P<0.05.
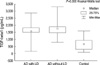
The mean age of patients with alcohol dependence and healthy controls were 45.2±9.2 and 48.3±10.1 yr, respectively (Table 1). There were no difference between patients of alcohol dependence and control subjects in age (P=0.15), in weight (P=0.13) and in height (P=0.84). As seen in Fig. 1, mean plasma TGF-β1 levels in the patients with alcohol dependence (1,653.11±532.45 pg/mL) were higher than in the control subjects (669.87±366.53 pg/mL) (P=0.000). Alcohol induced liver pathology were found with 16 patients after ultrasonography or computed tomography and consultation. Fatty liver was diagnosed in 2, alcoholic hepatitis was diagnosed in 8. Six turned out to be alcoholic cirrhosis patients. Comparison TGF-β1 level between alcohol dependence patients with or without liver pathology showed no difference (z=-0.91, P=0.36) (Fig. 2) However, when compared with control group, TGF-β1 levels showed significant difference (P=0.00) in alcohol dependence patients disregarding the presence or abseuce of liver pathology (P=0.00). Levels of TGF-β1 had no correlation with mean daily alcohol consumption, age of first use, and duration of dependency (P=0.97, P=0.79, P=0.77).
Our finding verified that TGF-β1 is increased also in human alcoholism. Mean plasma TGF-β1 levels in the patients with alcohol dependence were higher than in the control subjects. There had been an increased TGF-β1 mRNA in the patients with alcoholic hepatitis and alcoholic cirrhosis. But no difference had been found between alcohol abuser and normal controls (16). We found that alcohol dependence without liver pathology (no proof of fatty liver, hepatitis, cirrhosis with ultrasonography or computed tomography) had as high TGF-β1 as patients with liver pathology (Fig. 2). It may be possible to consider TGF-β1 increase as a predictor of alcohol induced liver damage.
Chronic alcohol drinking impairs intestinal barrier and decreases hepatic clearance, resulting in increased endotoxin levels in the liver (3, 17). These endotoxin bind with lipopolysaccharide (LPS)-binding protein which binds CD14 receptor at the Kupffer cell (18). Activated Kupffer cell releases TGF-β1 in paracrine pattern that activates hepatic stellate cell which is a major fibroblastic cell type in liver. In early stage hepatic stellate cell is highly responsive to TGF-β1 and transdifferentiate into myofibroblasts (19, 20). Receptor binded TGF-β1 activate Smad family which in turn translocate into nucleus and regulates target gene expressions (21).
Increased TGF-β1 induce hepatocyte apoptosis by producing oxidative stress in rat study (8). This effect is enhanced by ethanol stimulated CYP2E1 increase, especially in perivenous area of liver (22, 23).
Neuronal migration and neurite outgrowth are mediated by cell adhesion proteins (CAPs) such as neural cell adhesion molecule L1, and isoforms of integrin, which are regulated by TGF-β (24). Radial glia, which is a key element of guiding neuronal migration, mainly expresses TGF ligands and receptors (25-27) and also expresses cell adhesion proteins (28). TGF-β1 promotes CAP transcription and expression in low concentration, but at much higher concentration it decrease neuronal migration (12, 24). Thus, increased plasma TGF-β1 in alcoholism may suppress normal brain function.
Limitation of our study is that we checked only plasma TGF-β1 but did not checle the levels in cerebrospinal fluid. TGF-β1 does not cross the intact blood-brain barrier (29). However in normal condition only choroid plexus epithelial and meningeal cells express TGF-β1, but with brain lesion much induction occurs in other cell types (30). Another limitation of our study is that all of the subjects of our study were men. So we need a study in female patients to generalize our results.
Our result, increased plama TGF-β1 in alcohol dependence patients, suggests that alcohol induced liver damage and suppression of normal brain function may be mediated by TGF-β1. Alcohol induced liver fibrosis, heptocyte apoptosis, and neuronal suppression may be mediated by increased TGF-β1 in alcohol dependent patients. Further study about optimal suppression of TGF-β1 that can possibly prevent alcohol induced liver damage and cognitive impairment is needed.
Figures and Tables
References
1. Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005. 29(11):Suppl. 121S–131S.
2. Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003. 124:778–790.

4. Kwon OS, Song SH, Ju KT, Chung MG, Park DK, Kim SS, Kim YS, Koo YS, Kim YK, Choi DJ, Kim JH, Hwang YJ, Byun KS, Lee CH. Polymorphism in codons 10 and 25 of the transforming growth factor-beta1 gene in Korean population and in patients with liver cirrhosis and hepatocellular carcinoma. Korean J Gastroenterol. 2003. 42:212–219.
5. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000. 342:1350–1358.
6. Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997. 8:21–43.

7. Ravitz MJ, Wenner CE. Cyclin-dependent kinase regulation during G1 phase and cell cycle regulation by TGF-beta. Adv Cancer Res. 1997. 71:165–207.
8. Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002. 307:1–14.
9. Elliott RL, Blobe GC. Role of transforming growth factor beta in human cancer. J Clin Oncol. 2005. 23:2078–2093.

10. Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II recepor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002. 368:683–693.
11. Maehara Y, Kakeji Y, Kabashima A, Emi Y, Watanabe A, Akazawa K, Baba H, Kohnoe S, Sugimachi K. Role of transforming growth factor-beta 1 in invasion and metastasis in gastric carcinoma. J Clin Oncol. 1999. 17:607–614.
12. Luo J, Miller MW. Transforming growth factor beta1-regulated cell proliferation and expression of neural cell adhesion molecule in B104 neuroblastoma cells: differential effects of ethanol. J Neurochem. 1999. 72:2286–2293.
13. Miller MW, Luo J. Effects of ethanol and transforming growth factor beta (TGF beta) on neuronal proliferation and nCAM expression. Alcohol Clin Exp Res. 2002. 26:1281–1285.
14. Kuhn P, Sarkar DK. Ethanol induces apoptotic death of beta-endorphin neurons in the rat hypothalamus by a TGF-beta 1-dependent mechanism. Alcohol Clin Exp Res. 2008. 32:706–714.
15. Nanji AA, Tahan SR, Golding M, Khwaja S, Rahemtulla A, Lalani EN. Role of transforming growth factor-[beta]1 in inhibiting endothelial cell proliferation in experimental alcoholic liver disease. Am J Pathol. 1996. 148:739–747.
16. Chen WX, Li YM, Yu CH, Cai WM, Zheng M, Chen F. Quantitative analysis of transforming growth factor beta 1 mRNA in patients with alcoholic liver disease. World J Gastroenterol. 2002. 8:379–381.

17. Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002. 35:62–73.

18. Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002. 283:G256–G265.

19. Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000. 31:1094–1106.
20. Dooley S, Delvoux B, Streckert M, Bonzel L, Stopa M, ten Dijke P, Gressner AM. Transforming growth factor beta signal transduction in hepatic stellate cells via Smad2/3 phosphorylation, a pathway that is abrogated during in vitro progression to myofibroblasts. TGFbeta signal transduction during transdifferentiation of hepatic stellate cells. FEBS Lett. 2001. 502:4–10.
21. Stopa M, Anhuf D, Terstegen L, Gatsios P, Gressner AM, Dooley S. Participation of Smad2, Smad3, and Smad4 in transforming growth factor beta (TGF-beta)-induced activation of Smad7. THE TGF-beta response element of the promoter requires functional Smad binding element and E-box sequences for transcriptional regulation. J Biol Chem. 2000. 275:29308–29317.
22. Fang C, Lindros KO, Badger TM, Ronis MJ, Ingelman-Sundberg M. Zonated expression of cytokines in rat liver: effect of chronic ethanol and the cytochrome P450 2E1 inhibitor, chlormethiazole. Hepatology. 1998. 27:1304–1310.

23. Tsutsumi M, Lasker JM, Shimizu M, Rosman AS, Lieber CS. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989. 10:437–446.

24. Siegenthaler JA, Miller MW. Transforming growth factor beta1 modulates cell migration in rat cortex: effects of ethanol. Cereb Cortex. 2004. 14:791–802.
25. Flanders KC, Ludecke G, Engels S, Cissel DS, Roberts AB, Kondaiah P, Lafyatis R, Sporn MB, Unsicker K. Localization and actions of transforming growth factor-beta s in the embryonic nervous system. Development. 1991. 113:183–191.

26. Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J Physiol Paris. 2002. 96:25–30.
27. Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991. 115:1091–1105.

29. Kastin AJ, Akerstrom V, Pan W. Circulating TGF-beta1 does not cross the intact blood-brain barrier. J Mol Neurosci. 2003. 21:43–48.
30. Unsicker K, Strelau J. Functions of transforming growth factor-beta isoforms in the nervous system. Cues based on localization and experimental in vitro and in vivo evidence. Eur J Biochem. 2000. 24:6972–6975.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download