Abstract
We previously reported that nidogen is an extracellular matrix protein regulating Schwann cell proliferation and migration. Since Schwann cells play a critical role in peripheral nerve regeneration, nidogen may play a role in it via regulation of Schwann cells. Here, we demonstrate direct evidence that nidogen induces elongation of regenerative axon growth of adult sensory neurons, and that the effect is Schwann cell dependent. Continuous infusion of recombinant ectodomain of tumor endothelial marker 7, which specifically blocks nidogen function in Schwann cells, suppressed regenerative neurite growth in a sciatic nerve axotomy model. Taken together, it is likely that nidogen is required for proper regeneration of peripheral nerves after injury.
Nidogen is present in the basement membranes along with type IV collagen, laminin, and heparan sulfate proteoglycans (1, 2). Even though nidogen mediates the formation of the three-dimensional structure of the basement membranes as a structural protein (1, 3), nidogen also plays a role in cell attachment, chemotaxis of neutrophils and nerve development (4-8). In the peripheral nervous system (PNS), extracellular matrix (ECM) proteins play an important role in promoting nerve fiber growth during regeneration after injury (9). For example, laminin and fibronectin promote peripheral nerve regeneration (10, 11). However, the function of nidogen in peripheral nerve regeneration is still largely unknown.
During peripheral nerve regeneration, the ability of peripheral neurons to extend their axons is increased, and successful regenerative axon growth is also in part contributed by Schwann cells, a glial component in the PNS (9, 12, 13). Several mechanisms are implicated in the involvement of Schwann cells in peripheral nerve regeneration. First, Schwann cells secrete trophic factors for neurons (14) and secondly, Schwann cells seem to directly influence growing axons via process formation and migration (15, 16). The critical importance of Schwann cells in peripheral nerve regeneration was proved in experiments that showed great reduction of regeneration by the inhibition of Schwann cell proliferation and migration (17, 18).
We previously showed that a recombinant nidogen induces process formation and migration of Schwann cells (19). This finding led us to investigate whether nidogen regulates regenerative axon growth via modification of Schwann cell functions. In the present study, we examined the effect of nidogen on regenerative axon growth of adult dorsal root ganglion (DRG) neurons in vitro and in vivo.
For explant cultures in collagen gel, collagens were prepared as previously described (20). Thoracic and lumbar DRGs were removed from 40-50 days old male Sprague-Dawley rats (Samtako, Osan, Korea). Our college ethics committee approved that our protocol fulfilled the guide of animal experiments established by The Korean Academy of Medical Sciences (DIACUC-07-11). Following the dissection of a DRG into 2-3 explants under a stereomicroscope, the explants were implanted into collagen gel (Fig. 1) or placed on a 6 well-Millicell culture membrane (Millipore, Billerica, MA, U.S.A., 10-12 explants/membrane, Fig. 2) as described before (19). Then, Fc or nidogen-Fc (21) containing Dulbecco's modified Eagle's medium with 5% fetal bovine serum was added. After 2 or 3 days of culture, the explants were fixed with 4% paraformaldehyde and immunostained with an anti-Tuj1 antibody (1/2,000, Covance, Princeton, NJ, U.S.A.). The neurite outgrowth was examined under a Laser confocal microscope (Carl Zeiss, Hamburg, Germany). Quantification of the axonal elongation from the DRG explants (20 explants in each of three independent experiments) in collagen gel (Fig. 1) was carried out with LSM-510 software (Carl Zeiss) after photographing the explants under the Laser confocal microscope. Line draw tools were employed to automatically measure the length of the TuJ1-positive neurites (180-200 neurites per group), and the difference of mean length between groups was analyzed using the Student's t test. Parameters with values P<0.05 were considered to be different significantly.
Dissociated DRG neuronal cultures were performed according to standard protocol (22). Briefly, DRGs were enzymatically digested with 0.125% collagenase type I (Sigma, St. Louis, MO, U.S.A.) and 0.25% trypsin for 30 min at 37℃. After enzymatic digestion, the cells were dissociated by mechanical trituration through a fire-polished glass pipette. The cells (600 per well) were plated onto one well per eight-well plate (Nunc, Rochester, NY, U.S.A.) coated with Fc or nidogen-Fc, and cultured for 36-48 hr in the presence of cytosine arabinoside (10 µM). The neurites were quantified after immunostaining of the neurons with an anti-Tuj1 antibody. A cell with neurites longer than the cell body diameter was defined as a neurite-bearing cell, and the number of such cells was counted. The length of the longest neurite in a group of 180-200 neurons that were randomly selected was measured using an LSM 510 image analysis program (Carl Zeiss). The neurite lengths between the groups were compared using the Student's t test.
In order to study the role of nidogen in peripheral nerve regeneration in vivo, adult male Sprague-Dawley rats (150-300 g, n=10 for each group) were anesthetized with intraperitoneal injections of 10% ketamine hydrochloride (Sanofi-Ceva, Dusseldorf, Germany; 0.1 mL/100 g body weight) and 0.1 mL Rompun (Bayer, Leverkusen, Germany), and their sciatic nerves were exposed at mid-thigh level and sectioned with a fine iris scissor (FST Inc, Foster City, CA, U.S.A.). Infusion of recombinant proteins was performed by the method developed by Torigoe et al. (16) with minor modification. The proximal stump was sutured to a catheter (internal diameter, 0.72 mm) in parallel with a single epineurial 8-0 monofilament nylon suture. Both ends of the catheter and the proximal stump were placed into an artificial tube made by a Millicell membrane (Millipore) covered by a parafilm, and then fixed with sutures. The catheter was flushed with an Alzet mini-osmotic pump (Alzet, Cupertino, CA, U.S.A., model 1002; total content, 100 µL; delivery rate, 0.25 µL/hr for 14 days) containing alkaline phosphatase tag (AP) or ectodomain of tumor endothelial marker 7 (eTEM-AP [19]) (150 µg). Skin incision was closed with sutures, and animals were housed in plastic cages for 2 weeks.
The purification of Fc, nidogen-Fc, eTEM7-AP and AP was done as previously described (21).
Two weeks after the operation, the animals were sacrificed with cardiac perfusion. Briefly, the animals were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, and the proximal stump with the membrane tube was removed. The parafilm was separated from the membrane, and the membrane tube was carefully opened under a stereomicroscope. The membrane with regenerating proximal stump was immunostained with an anti-Tuj1 antibody and analyzed under a Laser confocal microscope (Carl Zeiss). The length of the regenerating neurites was measured and analyzed as described above. The thickness of the regenerating neurites was measured at the mid-point of randomly selected TuJi-positive neurites (-200 neurites per group) using LSM-510 software (Carl Zeiss), and the difference of mean thickness between groups was analyzed using the Student's t test. Parameters with values P<0.05 were considered to be different significantly.
We first examined whether the purified recombinant nidogen could induce regenerative axon growth of adult DRG explants using a classical three-dimensional culture. We cultured lumbar DRGs from adult rats for 36-40 hr in collagen gel containing Fc or recombinant nidogen (nidogen-Fc, n=20-30 explants for each group). In contrast to poor growth observed in the Fc-treated group, we observed extensive growth of Tuj1-positive neurites from explants containing nidogen-Fc (Fig. 1A), and the increase by nidogen-Fc was dose-dependent. We measured the mean length of neurites from explants, and found that the difference of the mean lengths between Fc and nidogen-Fc-treated group was statistically significant (P<0.05) at 20 µg/mL concentration (Fig. 1B). This experiment directly indicates the ability of nidogen to increase the neurite growth of adult DRG neurons in three dimensional cultures.
Since it was reported that nidogen was unable to increase neurite outgrowth in vitro (22), we suspected that the nidogen-induced axon growth of adult DRG explants might be mediated through Schwann cells. To address this issue, we employed a dissociated neuronal culture in which we could effectively eliminate proliferation of most Schwann cell population with cytosine arabinoside. We then analyzed the effect of nidogen on the neurite outgrowth of adult DRG neurons in dissociated cultures. As shown in Fig. 2, the number of neurite bearing cells from nidogen-Fc-coated dishes (up to 50 µg/mL) was not significantly different from that of Fc-coated dishes (Fig. 2B, P>0.05). We also examined the distribution of neurite length of the longest neurite within neurite-bearing cells, and found that it was not increased by nidogen-Fc (mean±SD, 271±54 µm, n=196 cells), compared to Fc-controls (254±78 µm, n=185 cells, P>0.05). In contrast, nidogen enhanced regenerative axon growth of DRG neurons in the absence of cytosine arabinoside (data not shown). This finding indicates that the increase of neurite outgrowth by nidogen might be dependent on Schwann cells.
We next employed a modified three dimensional culture that can easily monitor Schwann cell migration from DRG explants (Fig. 2C, [19]). Double immunostaining with an antibody to a neuron marker, Tuj1, and an antibody to S100 (a Schwann cell marker) allowed us to directly compare Schwann cell migration and neurite outgrowth on membrane dishes. In Fc-treated explants, minimal outgrowth of neurites and S100-positive cells were observed. In contrast, nidogen-Fc dramatically induced Schwann cell migration from DRG explants. Interestingly, an extensive outgrowth of neurites from explants was also observed in the nidogen-Fc-treated group. Furthermore, the distribution of neurites completely coincided with that of migrating Schwann cells (Fig. 2C). From these results, it can be inferred that nidogen-induced Schwann cell migration might participate in axonal outgrowth from DRG explants by nidogen in vitro.
In order to find out an endogenous role of nidogen in peripheral nerve regeneration, we tried to block nidogen using recombinant ectodomain of tumor endothelial marker 7 (eTEM7). We previously showed that TEM7 is a possible transmembrane receptor for nidogen and that eTEM7 can specifically block nidogen function on Schwann cells without affecting laminin or fibronectin functions (19). After sciatic nerve axotomy, we continuously infused recombinant eTEM-AP or AP for 2 weeks using an Alzet mini-osmotic pump. The immunostaining of regenerative axons from proximal stumps of the axotomized sciatic nerves demonstrated that the regenerating axons of the eTEM7-AP-treated group showed signs of abnormal regenerative axon growth, compared to that of AP-treated controls. First, the regenerative axons looked fragile and dystrophic in eTEM7-AP-treated group (Fig. 3A). Second, the diameter of the regenerating axons was thinner in eTEM7-AP-treated group, compared to the AP-control (Fig. 3B). However, the mean length of the regenerating axons in eTEM7-AP-treated group (Fig. 3C) was not significantly shorter than controls. These findings suggest that nidogen is required for proper regeneration of peripheral nerves after injury in vivo.
In the present study, we demonstrated that nidogen induces neurite growth of adult DRG neurons. Since the nidogen-induced neurite growth was not observed in the condition in which the growth of Schwann cells was suppressed, nidogen-induced changes of Schwann cells seem to contribute to the neurite elongation by nidogen. We previously reported that nidogen induces process formation and migration of Schwann cells in vitro (19). Schwann cell processes and migration are important for axonal regeneration of the axotomized nerves (15, 16). Regenerating axons extend from the proximal end in association with Schwann cell processes and appear to navigate along them (16). In agreement with this finding, we observed a correlation between Schwann cell migration and neurite outgrowth in explant cultures on a Millicell membrane. Thus the present study provides evidence to an interesting hypothesis that nidogen enhances neurite outgrowth from adult sensory neurons through Schwann cells.
It was previously shown that nidogen, co-purified with laminin, did not seem to have neurite promoting activity on neurons (22). In contrast to nidogen, laminin has a great potential to initiate neurite outgrowth in a variety of neuronal cells including DRG neurons (23). Thus it seems that nidogen has a different way to regulate neurite outgrowth from laminin, even though two proteins co-exist in the ECM of many tissues, including the peripheral nerves. However it is still unknown how nidogen alters the phenotype of Schwann cells to indirectly promote nerve regeneration. It may be possible that nidogen induces expression of cell adhesion molecules in Schwann cells, or nidogen may make Schwann cells release certain factors which can promote neurite growth. To clarify the factors which are induced by Schwann cells after their exposure to nidogen further studies are required including the molecular mechanism of this phenomenon.
We previously showed a dramatic increase of nidogen mRNA expression after sciatic nerve injuries (19). This finding may indicate a potentially important role of nidogens in peripheral nerve regeneration in vivo. In order to demonstrate an endogenous role of nidogen in peripheral nerve regeneration, we tried to specifically block nidogen during peripheral nerve regeneration using a recombinant ectodomain of TEM7. Even though the physiological relevance of the interaction between nidogen and TEM7 is still unknown, eTEM7 specifically inhibited Schwann cell responses to nidogen by interacting with nidogen (19). In this study, we found that continuous infusion of eTEM7 hampered the regenerative axon growth of injured sciatic nerves, compared to controls. The regenerating axons in the eTEM7-treated group were very thin and looked as if they were degenerating. Our in vivo experiment indicates that nidogen may be important for maintenance of the stability of regenerating axons. This finding in part coincides with findings of Torigoe et al. (16). They suggested that Schwann cells play a role in the late phase of nerve regeneration, rather than participating in the initial elongation of regenerating axons. On the other hand, the length of the regenerating axons was not shorter in eTEM7-treated group, compared to controls, indicating that nidogen might not be implicated in the initial elongation of regenerating axons in vivo, even though neurite outgrowth was induced by nidogen in explant cultures. If the dose of eTEM7 is increased, the length of regenerating axons may also be decreased. Taken together, our in vivo data support the hypothesis that nidogen might be involved in peripheral nerve regeneration through Schwann cells.
Conclusively, our observation for the first time attributes a functional role of nidogen to peripheral nerve regeneration. It is likely that nidogen can constitute a valuable therapeutic tool for peripheral nerve injuries.
Figures and Tables
Fig. 1
The effects of Fc and nidogen-Fc on the neurite outgrowth of adult DRG explants. (A) DRG explants from adult rats were cultured in collagen matrix, and the culture medium was supplemented with Fc (20 µg/mL) or nidogen-Fc (20 µg/mL) for 40 hr. Explants are immunostained with anti-Tuj1 to label axons. Scale bar; 100 µm. (B) Quantification of mean length of neurites. Values shown are mean lengths±standard deviation.
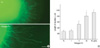
Fig. 2
Schwann cell-mediated neurite outgrowth by nidogen. (A) Dissociated neurons were purified from adult rat DRGs and cultured on Fc or nidogen-Fc-coated dishes for 36 hr in the presence of cytosine arabinoside, then immunostained with an anti-Tuj1 antibody. Representative images of the Fc-control and nidogen-Fc show no significant differences in neurite growth pattern. Scale bar; 10 µm. (B) Quantification of the number of neurite bearing cells in dissociated DRG cultures. (C) DRG explants were cultured on a Millicell membrane for 3 days, and then double immunostained with an antibody to Tuj1 (red) and an antibody to S100 (green). Note the significant colocalization of S100-positive Schwann cells and neurites in the nidogen-Fc treated group. Scale bar; 100 µm.
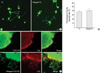
Fig. 3
In vivo effect of nidogen inhibition on peripheral nerve regeneration. After sciatic nerve axotomy, AP or eTEM7-AP was infused using an Alzet mini-pump for 2 weeks and then the regenerative axons were stained with an anti-Tuj1 antibody. (A) The axonal morphology is high dystrophic in the eTEM7-treated group compared to the Fc-treated group. Scale bar; 100 µm. (B). The diameter of neurites in eTEM7-AP-treated group was thinner than that of AP controls. *P<0.05. (C). The mean lengths of regenerating neurites from AP and eTEMP7-AP-treated group showed no difference.

References
1. Chung AE, Durkin ME. Entactin: structure and function. Am J Respir Cell Mol Biol. 1990. 3:275–282.

3. Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW. Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int. 1993. 43:7–12.

4. Chakravarti S, Tam MF, Chung AE. The basement membrane glycoprotein entactin promotes cell attachment and binds calcium ions. J Biol Chem. 1990. 265:10597–10603.

5. Dong L, Chen Y, Lewis M, Hsieh JC, Reing J, Chaillet JR, Howell CY, Melhem M, Inoue S, Kuszak JR, DeGeest K, Chung AE. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest. 2002. 82:1617–1630.

6. Kim S, Wadsworth WG. Positioning of longitudinal nerves in C. elegans by nidogen. Science. 2000. 288:150–154.
7. Senior RM, Gresham HD, Griffin GL, Brown EJ, Chung AE. Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-Asp (RGD) domain and the leukocyte response integrin. J Clin Invest. 1992. 90:2251–2257.

8. Yelian FD, Edgeworth NA, Dong LJ, Chung AE, Armant DR. Recombinant entactin promotes mouse primary trophoblast cell adhesion and migration through the Arg-Gly-Asp (RGD) recognition sequence. J Cell Biol. 1993. 121:923–929.
9. Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997. 14:67–116.

10. Bailey SB, Eichler ME, Villadiego A, Rich KM. The influence of fibronectin and laminin during Schwann cell migration and peripheral nerve regeneration through silicon chambers. J Neurocytol. 1993. 22:176–184.

11. Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003. 163:889–899.
12. Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005. 64:613–622.

14. Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992. 119:45–54.

15. Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995. 14:125–132.

16. Torigoe K, Tanaka HF, Takahashi A, Awaya A, Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp Neurol. 1996. 137:301–308.

17. Gulati AK, Rai DR, Ali AM. The influence of cultured Schwann cells on regeneration through acellular basal lamina grafts. Brain Res. 1995. 705:118–124.

18. Hall SM. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol Appl Neurobiol. 1986. 12:401–414.

19. Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 2007. 102:686–698.

20. Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004. 7:1222–1232.

21. Lee HK, Seo IA, Park HK, Park HT. Identification of the basement membrane protein nidogen as a candidate ligand for tumor endothelial marker 7 in vitro and in vivo. FEBS Lett. 2006. 580:2253–2257.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download