Abstract
The nonstinging house ant, Monomorium pharaonis (pharaoh ant), was recently identified as a cause of respiratory allergy. This study was performed to evaluate the extent of sensitization to pharaoh ant, and its clinical significance in asthmatic patients. We carried out skin prick tests in 318 patients with asthma. Specific IgE (sIgE) to pharaoh ant was measured by ELISA, and cross-reactivity was evaluated by ELISA inhibition tests. Bronchial provocation testing was performed using pharaoh ant extracts. Fifty-eight (18.2%) of 318 patients showed positive skin responses to pharaoh ant, and 25 (7.9%) had an isolated response to pharaoh ant. Positive skin responses to pharaoh ant were significantly higher among patients with non-atopic asthma than among those with atopic asthma (26.0% vs. 14.9%, p<0.05). There was significant correlation between sIgE level and skin responses to pharaoh ant (rho=0.552, p<0.001). The ELISA inhibition tests indicated that pharaoh ant allergens had various pattern of cross-reactivity to house dust mites and cockroaches. Bronchial provocation tests to pharaoh ant were conducted for 9 patients, and eight showed typical asthmatic reactions. In conclusion, pharaoh ant is an important source of aeroallergens, and it should be included in the skin test battery for screening the causative allergens in patients with asthma.
Several studies indicate that insects are significant source of aeroallergens (1-5). Although cockroaches have been extensively studied, other insects have been rarely evaluated as a source of indoor inhalant allergens (6-8). Changes of life style and living environment have encouraged the growth of arthropods including house dust mites (HDMs) and insects, and have increased allergen exposure. Thus, recognition of unknown avoidable allergens is an important part of clinical allergy practice.
The nonstinging house ant, Monomorium pharaonis (pharaoh ant), is a highly infesting species in the urban indoor environment. We recently identified that the pharaoh ant can act as a source of aeroallergens (9). Despite of its possible role in respiratory allergy, no detailed study has been performed whether pharaoh ant represents an important source of inhalant allergens. We performed this study to evaluate type I hypersensitivity to pharaoh ant and its clinical significance in respiratory allergy. This is the first study in which large number of patients with bronchial asthma was evaluated for sensitization to pharaoh ant, and for clinical and immunologic characteristics of pharaoh ant allergy.
A total of 318 young and middle-aged adult patients with bronchial asthma aged from 15 to 50 yr (mean 30.8 yr, 192 males, 126 females) who visited Allergy Clinic of Yonsei University Severance Hospital between January 2001 and December 2002 were enrolled in this study. Asthma was diagnosed if patient with typical symptoms of asthma showed either reversible airflow limitations defined as improvement of FEV1 of more than 15% after inhalation of 200 µg of salbutamol, and/or airway hyperresponsiveness to methacholine. Steroid-dependent asthmatics or patients with associated cardio-pulmonary disorders were excluded in the present study. Ten healthy subjects (6 males, 4 females) with no personal or family history of allergic disorders were also studied as controls.
Pharaoh ants were collected from the patients' houses, and their species were confirmed by Professor B. J. Kim (Wonkwang University, Iksan, Korea). They were ground up and extracted into phosphate-buffered saline solution (PBS, pH 7.4) 1:20 w/v at 4℃ for 48 hr, followed by centrifugation at 12,000 rpm for 1 hr. The supernatant was dialyzed against distilled water at 4℃ for 48 hr and then used as crude extract. The protein concentration of the extract was 500.3 µg/mL, as determined using the BioRad method. For skin prick testing, the extract was diluted with PBS and then mixed with an equal amount of sterile glycerin (final concentration of 1:100 w/v).
Skin prick tests were performed using 1 mg/mL histamine as a positive control, the diluent as a negative control, pharaoh ant extract (1:100 w/v), imported fire ant (IFA) extract (Hollister-Stier Laboratories LLC, Spokane, WA, U.S.A.), and 30 common aeroallergens including pollens, molds, animal danders, cockroaches, Dermatophagoides farinae, and Dermatophagoides pteronyssinus (AT Ltd, Worthing, West Sussex, U.K.). After 15 min, the mean diameter of the wheal formed by the allergen was compared with that formed by histamine. If the former was the same or larger than the latter (A/H ratio ≥1), it was considered positive. Atopy was defined as positive skin response to one or more inhalant allergens among 30 common aeroallergens.
The presence of specific IgE (sIgE) to pharaoh ant was determined by using the ELISA according to the method described previously (9). Microtiter plates were coated with 50 µL of 0.05 M carbonate buffer (pH 9.6) containing 10 µg/mL of pharaoh ant extract at 4℃. After blocking, the plate was incubated with 50 µL of either patient or control sera (undiluted) from 10 healthy volunteers with negative skin prick test responses to common aeroallergens as well as to pharaoh ant. After washing, the plate was incubated with 50 µL of biotinylated anti-human IgE (Vector Laboratories, Burlingame, CA, U.S.A.) followed by incubation with streptavidin-peroxidase (Sigma-Aldrich Corp, St Louis, MO, U.S.A.). After washing, 100 µL of ABTS solution (25 mg 2,2'-azino-bis-3-ethylbenzthiazoline-sulfonic acid in 50 mL of 50 mM citrate phosphate buffer containing 50 µL of 0.03% hydrogen peroxide) was added as a substrate, and after 5 min 100 µL of 2 mM NaN3 was added to stop the reaction. A colorimetric reaction was measured by the absorbance at 405 nm on an ELISA reader (Dyna-tec, Alexandria, CA, U.S.A.). The positive cutoff value was decided as mean +2×SD of the absorbance value of the healthy control serum samples.
To determine the specificity of the IgE binding and the allergenic cross-reactivity, ELISA inhibition tests were performed. The patient's undiluted serum was preincubated with inhibitors such as IFA (Bayer, Pittsburgh, PA, U.S.A.), Pachycondyla chinensis, Blattella germanica, and D. farinae extracts (supplied from Korea National Research Resource Collection of Allergens, Seoul, Korea), and then added to a pharaoh ant antigen-coated immunoplate. The same steps were followed as in the ELISA.
In order to evaluate the specificity of sIgE response to pharaoh ant, specific bronchial provocation tests (BPTs) were performed according to the method previously described (9). Briefly, patients inhaled the aerosolized extract dissolved in 0.4% phenol/0.9% saline for 2 min by using the tidal breathing method. A control challenge with phenol-saline was performed before antigen provocation, and then serial increments of ant extracts (from 1:100,000 to 1:1,000 w/v) were administered at 10-min intervals until a 20% or greater decrease in FEV1 was obtained or until the highest concentration of the extract was inhaled. Both FEV1 and FVC were measured every 10 min during the first hour, and then every hour for the remaining 7 hr after the challenge.
Statistical analysis was performed using the chi-square tests, and Student's t-tests to assess differences between positive skin responders and negative responders. Spearman's rank correlation was calculated to assess the correlation between data. A p-value of 0.05 or less was regarded as significant.
A total of 222 out of 318 patients showed positive responses to one or more inhalant allergens, and the rate of atopic asthma was 69.8% in this study. Fig. 1 shows skin prick test responses to common inhalant allergens and pharaoh ant. D. farinae was the most common sensitizing allergen (52.8%), followed by D. pteronyssinus (52.5%), cockroach (27.4%), and cat (19.8%). Among the 318 enrolled patients, 58 (18.2%) elicited a positive reaction to pharaoh ant, and skin sensitization to pharaoh ant was much higher than to other important aeroallergens in Korea (dog 15.1%, mugwort pollen 11.3%, ragweed pollen 8.5%, birch pollen 5.7%, etc). Skin prick test revealed that 25 (7.9%) had an isolated positive response to pharaoh ant with negative results to other common aeroallergens in their environment. The positive skin responses to pharaoh ant were significantly higher among patients with non-atopic asthma (25 of 96, 26.0%) than among those with atopic asthma (33 of 222, 14.9%) (p=0.018).
Table 1 shows the clinical characteristics of the patients according to skin responses to pharaoh ant. No significant difference of age, sex, level of total IgE, and degree of airway hyperresponsiveness to methacholine was noted between positive skin responders and negative responders to pharaoh ant. The positive skin responses to house dust mites, cockroach, cat, mugwort, and other common aeroallergens were not higher among subjects with positive responders than among those with negative responders to pharaoh ant. Despite higher tendency of sensitization to IFAs in subjects with positive responders, no statistical significance of positive skin responses to IFAs was noted between positive responders and negative responders to pharaoh ant.
Levels of sIgE to pharaoh ant were measured with sera of the 58 patients with positive skin response to pharaoh ant, 50 randomly selected patients with negative skin response to pharaoh ant, and 10 controls by using ELISA method. Fifty (86.2%) of the 58 patients with positive skin responders had significant level of sIgE to pharaoh ant, on the other hand, 5 (10%) of 50 negative skin responders had sIgE. None of the controls gave a positive sIgE to pharaoh ant (Fig. 2). Thus 50 (15.7%) of 318 enrolled patients showed both positive skin test reactions and sIgE antibodies to pharaoh ant. A significant correlation was noted between levels of sIgE and skin responses (allergen/histamine ratio) to pharaoh ant (rho=0.552, p<0.001, Fig. 3) in patients with positive skin responders.
To determine the allergenic cross-reactivity of pharaoh ant, we performed ELISA inhibition tests with sera of patients with high sIgE to pharaoh ant. In many patients, the results of ELISA inhibition testing indicate that sIgE binding to pharaoh ant was completely inhibited by pharaoh ant, partially inhibited by IFA antigen, but not inhibited by P. chinensis, B. germanica, and D. farinae extract (Fig. 4A). On the other hand, some patients showed different pattern of cross-reactivity; IgE binding to pharaoh ant was partially inhibited by B. germanica, and D. farinae extract, but not by P. chinensis (Fig. 4B).
BPTs were performed on 9 patients living in a home with visual evidence of ant infestation with positive skin reaction and high sIgE to pharaoh ant. Of them, 8 patients showed typical pattern of asthmatic reactions (4 showed isolated early asthmatic reactions, 4 dual asthmatic reactions). These 8 patients were considered as having pharaoh ant-allergic asthma based on their environmental characteristics, positive skin test and sIgE, and positive response to BPTs with pharaoh ant extract. BPTs with pharaoh ant extract were also performed on 5 patients with negative skin testing and sIgE response, but no significant change in FEV1 was noted.
Table 2 shows the clinical characteristics of patients with pharaoh ant-sensitive asthma. All had lived in urban areas and experienced asthmatic symptoms as well as recurrent nasal symptoms. Four patients (No. 1, 3, 5, and 6 in Table 2) were housewives and spent plenty of time in their home, and remain four were students spending a lot of time in their homes or schools. None had any pet or household mold problems at their homes, and had known history of ant sting or bite injury. On skin prick test, 4 had an isolated positive response to pharaoh ant with negative results to common aeroallergens. All patients were recommended to eradicate ant infestation at their homes by measures such as restricting access, chemical control, and traps. Treatment with such environmental control and pharmacological treatment led progressive improvement of symptoms in all patients.
Development of immunochemical assay can measure various air-borne allergens irrespective of their morphological features. Using various techniques, insect-related particles of less than 10 µm in size have been demonstrated in the air or dust samples (10-12). Therefore, the significance of insects as a source of inhalant allergens has been widely accepted. Several studies have reported that many kinds of insects including locusts, crickets, moths, houseflies can cause respiratory sensitization, but the clinical significance of IgE to these insects remains obscure owing to lack of clinical correlation (2-5). Furthermore insects other than cockroaches have been rarely evaluated as a source of indoor aeroallergens (6-8). We recently reported that the nonstinging house ant, pharaoh ant, can act as an indoor source of inhalant allergens (9). To eliminate possible confounding factors, old patients with asthma were excluded and only young and middle-aged adults with asthma were included in this study. It was found that many patients with asthma have sIgE response to pharaoh, and some patients selected for BPTs showed typical asthmatic reactions after inhalant exposure to pharaoh ant antigens. These results indicated that pharaoh ant is an important source of indoor inhalant allergens. This is the first study that the clinical significance of pharaoh ant was evaluated in large number of patients with asthma.
The ant is a widespread insect that comprises 11 subfamilies, 297 genera, and approximately 8,800 species (13). In Korea 4 subfamilies, 33 genera, and 104 species of ants have been registered, and the 4 subfamilies include the Formicinae, the Myrmicinae, the Ponerinae, and the Dolichoderinae (14). Pharaoh ant, the tiny yellow ant belonging to the Myrmicinae subfamily and the Formicidae family, is of tropical origin and do not nest out of doors except in southern latitudes (Fig. 5). It has become a seriously troublesome insect in indoor environment, and its infestation has continuously increased because of its ability to disperse (13, 14). In recently performed our field survey, it was found that pharaoh ant infests in about 20% of homes in Seoul, Korea (unpublished data).
There is little data on the prevalence of sensitization to ant. Lierl et al. screened children with allergic asthma by RAST for sIgE directed against outdoor insects, and found prevalence of sensitization to ant up to 12.9% (1). Although they did not clearly state the scientific name of the ant, they used outdoor ant. In current outdoor environment, it is difficult that particles derived from non-flying outdoor ant can be airborne enough to be exposed to human via respiratory route. Moreover, about 20% of adults living in a fire ant endemic area showed positive skin response and sIgE to fire ant, debating on the significance of the presence of sIgE to outdoor ant in population (15). The clinical meaning of sIgE to ant in previous study remains more and more uncertain because many of the subjects reacted with more than one insect species, implying extensive cross-sensitization (1). There is good evidence that pan-allergens like tropomyosin are responsible for this multiple reactivity (16, 17).
However, pharaoh ant in this study is indoor ant, and pharaoh ant-derived particles such as shed scale, excreta, and disintegrated bodies are more easily accumulated in environmental reservoirs and exposed to human in indoor environment than in outdoor environment. Some patients in this study had an isolated positive response to pharaoh ant with negative results to other aeroallergens, and this result also suggests that response to pharaoh ant represent not cross-reactive but specific responses. The results of ELISA inhibition tests showing complete inhibition of IgE binding by pharaoh ant itself but not by other indoor allergens indicate that sIgE response to pharaoh ant is specific. A concept of insect pan-allergy is opposed by reports similar to the present one suggesting that sensitization may be more specific in patients with insect allergy (5, 18). After considering all these findings, we strongly suggest that pharaoh ant allergens have unique allergenic determinants, and elicit sIgE-mediated immune reactions.
Allergen sensitization is known to occur at a higher rate among atopic individuals (19, 20). In this study, positive skin responses to common aeroallergens such as HDMs, cockroaches, animal dander, and pollens were not different between subjects sensitized to pharaoh ant and those not sensitized. This finding suggest that sensitization to pharaoh ant is influenced by factors other than genetic predisposition to atopy alone. Some environmental factors may affect sensitization to pharaoh ant. However we did not get detailed information on home environment such as family size, housing type, location of home, presence of ant infestation or pet, and so on from individual subject. Thus it is not known which environmental factors may affect sensitization to pharaoh ant, and further study may be required for this important issue.
The recognition of allergic sensitizations may determine the prognosis and treatment of asthma. Detection of removable sources of allergens is particularly significant in clinical practice. Some patients diagnosed as having non-atopic asthma may be sensitized to unknown allergens. In this study, a quarter of adult patients with non-atopic asthma were, in fact, sensitized to pharaoh ant. We strongly suggest that pharaoh ant allergens should be included in skin test batteries of common inhalant allergens for screening the causative allergens in patients with asthma.
Although recent consensus reports stress the importance of environmental control for allergen, the clinical benefit of allergen avoidance is still controversial. Effect of allergen avoidance in adults is more complicated by several factors such as presence of chronic obstructive pulmonary disease, effect of smoking and pollutants, and the problem of airway remodeling (21). Although occupational asthma is frequently used as a model explaining the association between cessation of exposure and asthma prognosis, delayed diagnosis and subsequent irreversibility of occupational asthma may lead to falsely unfavorable outcomes. We already reported two cases of pharaoh ant-sensitive asthma who showed remission of asthma and negative conversion of airway hyperresponsiveness after allergen avoidance (9). We think that pharaoh ant-sensitive asthma is a good model for the study of the allergen avoidance. Therefore further longitudinal studies will clearly demonstrate the benefit of allergen avoidance on patients with bronchial asthma.
The high frequency of skin reactivity along with the results of sIgE ELISA and BPT in this study suggest that the pharaoh ant-derived particles possess potent allergenic components and are present in the indoor environment in sufficient quantities and for a sufficient time to cause sensitization. In conclusion, pharaoh ant is an important indoor source of inhalant allergens among patients with asthma living in urban areas, and pharaoh ant allergens have unique allergenic determinants. These species should be taken into consideration as a cause of asthma in patients living in urban areas, especially in homes with visual evidence of ant infestation.
Figures and Tables
Fig. 1
Positive skin responses to common inhalant allergens. DF, Dermatophagoides farinae; DP, Dermatophagoides pteronyssinus; P. ant, pharaoh ant.

Fig. 2
Specific IgE in serum binding to pharaoh ant in positive, negative skin responders to pharaoh ant, and healthy controls. Horizontal dotted line indicates the positive cut-off value of specific IgE to pharaoh ant, which were derived from mean+2×SD of the absorbance values of the healthy controls.

Fig. 3
Relationship between levels of specific IgE antibodies and skin responses to pharaoh ant in patients with positive skin responses to pharaoh ant.
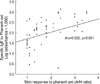
Fig. 4
Enzyme-linked immunosorbent assay inhibition of pharaoh ant specific IgE. Individual serum samples were preincubated with inhibitors [pharaoh ant (●), imported fire ant (▲), P. chinensis (◆), D. farinae (■), B. germanica (○)] and then added to a pharaoh ant-coated immunoplate. Specific IgE binding to pharaoh ant is inhibited by pharaoh ant, but not by P. chinensis, B. germanica, and D. farinae extract (A). Serum from some patients showed different pattern of cross-reactivity; specific IgE binding is partially inhibited by B. germanica, and D. farinae extract, but not by P. chinensis (B).

Table 2
Clinical features of the patients with pharaoh ant-sensitive asthma

skin response, skin response to pharaoh ant; A/H, wheal size formed by pharaoh ant/histamine; sIgE, pharaoh ant-specific IgE, levels are described as absorbance value in ELISA; BPT, pharaoh ant-specific bronchial provocation test; PC20, provocative concentration of methacholine resulting in 20% fall in FEV1; Early, early asthmatic reaction; Dual, dual asthmatic reaction; Oak, oak pollen; Birch, birch pollen.
ACKNOWLEDGEMENTS
The photo of Monomorium pharaonis was taken by Lee IY, Ph.D. (Department of Parasitology, Yonsei University College of Medicine, Seoul, Korea). These ants were caught by Mrs. Kim YS in her home (Incheon, Korea) and their species were identified by Prof. Kim B-J (Wonkwang University, Iksan, Korea).
References
1. Lierl MB, Riordan MM, Fischer TJ. Prevalence of insect allergen-specific IgE in allergic asthmatic children in Cincinnati, Ohio. Ann Allergy. 1994. 72:45–50.
2. Tee RD, Gordon DJ, Hawkins ER, Nunn AJ, Lacey J, Venables KM, Cooter RJ, McCaffery AR, Newman Taylor AJ. Occupational allergy to locusts: an investigation of the sources of the allergen. J Allergy Clin Immunol. 1988. 81:517–525.

3. Bagenstose AH III, Mathews KP, Homburger HA, Saaveard-Delgado AP. Inhalant allergy due to crickets. J Allergy Clin Immunol. 1980. 65:71–74.
4. Komase Y, Sakata M, Azuma T, Tanaka A, Nakagawa T. IgE antibodies against midge and moth found in Japanese asthmatic subjects and comparison of allergenicity between these insects. Allergy. 1997. 52:75–81.

5. Focke M, Hemmer W, Wohrl S, Gotz M, Jarisch R, Kofler H. Specific sensitization to the common housefly (Musca domestica) not related to insect panallergy. Allergy. 2003. 58:448–451.

6. van Wijnen JH, Verhoeff AP, Mulder-Folkerts DK, Brachel HJ, Schou C. Cockroach allergen in house dust. Allergy. 1997. 52:460–464.

7. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997. 336:1356–1363.

8. Lewis SA, Weiss ST, Platts-Mills TA, Burge H, Gold DR. The role of indoor allergen sensitization and exposure in causing morbidity in women with asthma. Am J Respir Crit Care Med. 2002. 165:961–966.

9. Kim CW, Choi SY, Park JW, Hong CS. Respiratory allergy to the indoor ant (Monomorium pharaonis) not related to sting allergy. Ann Allergy Asthma Immunol. 2005. 94:301–306.

10. Swanson MC, Agarwal MK, Reed CE. An immunochemical approach to indoor aeroallergen quantitation with a new volumetric air sampler: studies with mite, roach, cat, mouse, and guinea pig antigens. J Allergy Clin Immunol. 1985. 76:724–729.

11. Kino T, Chihara J, Fukuda K, Sasaki Y, Shogaki Y, Oshima S. Allergy to insects in Japan. III. High frequency of IgE antibody responses to insects (moth, butterfly, caddis fly, and chironomid) in patients with bronchial asthma and immunochemical quantitation of the insect-related airborne particles smaller than 10 microns in diameter. J Allergy Clin Immunol. 1987. 79:857–866.
12. Wynn SR, Swanson MC, Reed CE, Penny ND, Showers WB, Smith JM. Immunochemical quantitation, size distribution, and cross-reactivity of lepidoptera (moth) aeroallergens in southeastern Minnesota. J Allergy Clin Immunol. 1988. 82:47–54.

13. Holldobler B, Wilson EO. The ants: classification and origins. 1990. Cambridge (MA): The Belknap Press of Harvard University Press;4–141.
14. Choi BM, Masaki K, Choi MK. Study on distribution of ants (Formicidae) from Korea (II): formic fauna in Mt. Halla [in Korean]. Cheong Ju Tea Coll. 1985. 22:439–462.
15. Hoffman DR, Dove DE, Moffitt JE, Stafford CT. Allergens in Hymenoptera venom. XXI. Cross-reactivity and multiple reactivity between fire ant venom and bee and wasp venoms. J Allergy Clin Immunol. 1988. 82:828–834.

16. Witteman AM, Akkerdaas JH, van Leeuwen J, van der Zee JS, Aalberse RC. Identification of a cross-reactive allergen (presumably tropomyosin) in shrimp, mite and insects. Int Arch Allergy Immunol. 1994. 105:56–61.

17. Panzani RC, Ariano R. Arthropods and invertebrates allergy (with the exclusion of mites): the concept of panallergy. Allergy. 2001. 56:Suppl 69. 1–22.

18. Gupta S, Jain S, Chaudhry S, Agarwal MK. Role of insects as inhalant allergens in bronchial asthma with special reference to the clinical characteristics of patients. Clin Exp Allergy. 1990. 20:519–524.

19. Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998. 101:587–593.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download