Abstract
The effect of genistein on aortic atherosclerosis was studied by immunohistochemistry with RAM-11 and HHF-35 antibodies and western blotting for matrix metalloproteinase-3 (MMP-3) in New Zealand White rabbits. After provocation of atherosclerosis with hyperlipidemic diet, the rabbits were divided as hyperlipidemic diet group (HD), normal diet group (ND) and hyperlipidemic plus genistein diet group (HD+genistein) for 4 and half months. The average cross sectional area of atherosclerotic lesion was 0.269 mm2 after provocation. The lesion was progressed by continuous hyperlipidemic diet (10.06 mm2) but was increased mildly by genistein (0.997 mm2), and decreased by normal diet (0.228 mm2). The ratio of macrophages to smooth muscle cells in the lesion was not changed by genistein supplementmentation. The western blotting showed reduction of MMP-3 expression in HD+genistein and ND groups than HD group. The inhibition of atherogenesis by genistein was might be due to improve the endothelial dysfunction rather than direct action on macrophages and/or smooth muscle cells in the lesion, since endothelial dysfunction by lipid peroxidation was the main atherogenic factor in the hypercholesterolemicrabbits. The genistein supplementmentation also suggests that it helps the stabilization of the atherosclerotic lesion by inhibition of MMP-3 expression.
The low mortality with cardiovascular disease in women is partly due to estrogen, which has many biologic effects including anti-atherogenic action. Phytoestrogen, a kind of soyprotein, has a weak but similar action with estrogen on atherosclerosis. Adlercreutz (1) suggests that populations that consume a high phytoestrogen diet have a lower risk of cardiovascular disease and cancer. The lower incidence of cardiovascular disease in Asian countries and in vegetarians suggests that phytoestrogens may be cardioprotective.
Genistein is a principal isoflavone found in soy phytoestrogen, and it has structural similarity with 17β-estradiol. Phytoestrogen shows many anti-atherogenic activities. It decreases cholesterol concentration (2), lowers blood pressure (3) and increase HDL cholesterol (4). Genistein inhibits vascular smooth muscle cell proliferation (5), improves arterial compliance (6, 7) and it has an antioxidant action (8, 9). Therefore, dietary phytoestrogen may inhibit atherosclerosis through these actions.
Studies of clinical events with atherosclerosis provide that plaque stability seems a more important factor than lesion size (10). Activated T lymphocytes in atherosclerotic lesion produce an inflammatory cytokine interferon-γ (IFNγ). IFNγ acts on vascular smooth muscle cells to decrease the synthesis of interstitial collagen (11, 12). IFNγ also inhibits smoothmuscle cell proliferation (13), helping reduction of collagen synthesis in the atheromatous lesion. In addition, collagen breakdown by the proteolytic enzymes, such as metalloproteinases (MMP) family, further weaken the fibrous cap (14, 15).
Since macrophages and smooth muscle cells are major cells in atherosclerotic lesion, we are interested in the relationship between the dietary intake of genistein and composition of macrophages and smooth muscle cells in the lesion. In fact, lipid lowering reduces the number of macrophages in experimental atherosclerosis and stabilizes the plaques by reducing proteolytic activity (16, 17).
In this study, we investigated the effect of dietary genistein on hypercholesterol diet-induced atherogenesis in rabbit aortas. We studied the effects of genistein supplementmentation on lesion progression, ratio of macrophages to smooth muscle cells using immunohistochemistry. We also measured matrix metalloproteinase-3 expression by western blotting in the aortic wall.
Twenty eight young male New Zealand White rabbits aged 5 weeks were obtained from Hanil Animal Laboratory Co. (Wanju, Korea). Rabbits were individually housed in metal cages in an air-conditioned room (22±1℃). Rabbits were fed Purina® rabbit diet (Agribrands Purina Korea, Korea) for 2 weeks for adaptation. Rabbits had free access to tap water. After two weeks, all rabbits were isolated and fed hypercholesterol diet (HD: cholesterol 0.5% w/w; Sigma Chemical, St. Louis, MO, U.S.A.). The diet was prepared as pellet and continued for 4 months for provoking atherosclerosis. After provoking, 7 animals were euthanized for lesion evaluation and remaining 21 animals were randomly divided into 3 groups: (1) 7 on ND, (2) 7 on HD, and (3) 7 on HD plus Genistein diet (Genistein 1.65 mg/kg of rabbits; Oncogene, Fremont, CA, U.S.A.). Rabbits were fed the respective diets for another 4 and half months. At the end of 4 and half months all animals were sacrificed for evaluation of the aorta (Table 1).
The aortas were removed and both proximal and distal 1 cm of the aorta were freezed by liquid nitrogen and stored at -80℃ for molecular study. Others were fixed in 10% buffered formalin solution. Two sections of 0.5 cm-length each from the proximal, middle and distal portion (total 3 sections) were embedded in paraffin (Paraplast, Oxford, St. Louis, MO, U.S.A.) for light microscopy and immunohistochemistry. Sections taken from each artery were stained with Verhoeff-Van Gieson staining (18) and evaluated the development of atherosclerotic lesion by image analyzer using Visus Image Analysis System (Image and Microscopy Technology, Korea). In short, we measured the round area consist of boundary line along the intimal elastic lamina, and then we subtracted the luminal area from each area. We considered it was a lesion area. The lesion size was calculated from nine sections (three sections from each block), and expressed as mean±SD.
Sections taken from each artery were evaluated for RAM 11 (DAKO Corporation, Carpinteria, CA, U.S.A.) and HHF-35 (DAKO) expression using an each monoclonal antibodies. Simultaneous staining of slides from all groups has performed. Four µ-thick paraffin sections from the arteries were incubated with 3% hydrogen peroxide for five minutes, then with 0.5% casein-Tris buffer for thirty minutes for blocking nonspecific binding. The slides were incubated with anti-RAM 11 and HHF-35 for two hours. After incubation with species appropriate biotinylated secondary antibodies (Vector Laboratories, Vectorstain ABC kit; Vector Laboratories, Burlingame, CA, U.S.A.) for 30 min, washed and incubated with streptavidin conjugated horseradish peroxidase (HRP) for 30 min. HRP visualization was carried out using 3,3'-diaminobenzidine tetrahydrochloride (ScyTek, Logan, Utah, U.S.A.) as substrate. Sections were counterstained with Light Green solution. Slides in which primary antibodies were omitted served as negative controls for each antibody used in this study.
The brown-stained areas by anti-RAM 11 and anti-HHF-35 antibodies were regarded as macrophages and smooth muscle cell, respectively, and measured by the image analyzer.
Each tissue were homogenized in ice-cold buffer of 50 mM Tris-HCl, pH 7.4/1 mM EDTA containing antipain (10 µg/mL), leupeptin (10 µg/mL), and phenylmethylsulfonyl fluoride (100 µg/mL), and centrifuged at 4℃, 12,000 rpm for 30 min and supernatant collected. Protein content in each sample was determined by a BioRad protein assay. Twenty µg of protein in each were removed and mixed with 6×sodium dodecyl sulfate (SDS) sample buffer (4×Tris-HCl/SDS pH 6.8, glycerol (20%), SDS (1%), bromophenol blue (1.2%), 3 M Urea). The sample mixture was boiled at 100℃ for 5 min and loaded onto a 6% polyacrylamide mini-gel for SDS polyacrylamide gel electrophoresis (SDS-PAGE). Proteins separated on the minigel were transferred onto a nitrocellulose membrane with a Bio-Rad transfer system at 80 V for 1 hr. The membrane was blocked with 5% nonfat dry milk in PBS at room temperature for 1 hr and incubated at 4℃ overnight with mouse monoclonal antibody against rabbit matrix metalloproteinase-3 (Oncogene, U.S.A.) diluted at 1:1,000 in blocking buffer. After washing with PBS containing 0.1% Tween-20, the membrane was incubated with a horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody at room temperature for 1 hr. The membrane was washed with PBS, developed by an enhanced chemiluminescence (Amersham Pharmacia Biotech, Buckinhamshire, U.K.) western blot analysis system and exposed to Kodak XAR radiography film.
The lesions were developed mildly during the provocation period (average: 0.269 mm2), however, no aortas revealed any gross or microscopic atherosclerotic lesion in the control group. On light microscopic examination, the lesions consisted of intimal hyperplastic lesion, which mainly composed of lipidladen macrophages (foam cell) and smooth muscle cells. There were some variation of the lesions, but any fibrotic, calcific, or thromobotic plaque lesion was not demonstrated. No morphologic differences were demonstrated among experimental groups. All cholesterol-fed animals (ND, HD+G and HD group) had a lesion on the aortic endothelium (Fig. 1).
The lesion is progressed by continuous hyperlipidemic diet (average: 10.06 mm2), which is prevented partly by genistein (average: 0.997 mm2). Normal diet decreased the lesion size (average: 0.228 mm2).
The immunohistochemistry for RAM-11 revealed diffuse positive reaction in foamy cells and histiocytes in the lesion (Fig. 2). The characteristic of the reaction was not different among three experimental groups. The reactions with HHF-35 antibody showed in the smooth muscle cells within the lesion (Fig. 3). However, the staining intensities were slightly weaker than RAM-11, although it revealed similar reaction among the experimental groups. The average areas of positive reaction to each antibody were measured by the image analyzer, and the ratio of RAM-11 to HHF-35 area was measured (Fig. 4). There was no difference of statistical significance between the experimental groups (p>0.05).
ND and HD+G groups show similar bands of intensity on Western blotting with MMP-3. However, HD group revealed stronger expression of MMP-3 (Fig. 5).
The genistein supplementmentation inhibits the atherogenesis and decreases the MMP-3 expression in the aorta. However, it has no or little effect on the ratio of macrophages/smooth muscle cells in the aortic atherosclerotic lesions.
Phytoestrogen has been known to have many anti-atherogenic actions as described in the introduction, but there are also some inconsistent reports on the action of phytoestrogen on blood lipid concentration. Probably that is originated from variable intestinal absorption in each experimental models and/or different concentration of phytoestrogen in the diets or materials. However, phytoestrogen or estrogen may have bigger action in improving vascular functions than in just reducing cholesterol (19-22), and this is independent of estrogen's action on plasma lipids (20). Therefore, such action may be a main mechanism in the decrease in ischemic heart disease in women treated with estrogen replacement (21).
Lipid peroxidation due to hyperlipidemic diet may be the predominant factor of endothelial injury in this hypercholesterolemic model. Therefore, the inhibition of the atherosclerotic lesion by genistein might be due to the improvement of the endothelial dysfunction with decreasing oxidative modification of LDL cholesterol. Squadrito et al. (23) showed that genistein supplementmentation improves endothelial dysfunction in ovariectomized rats.
It is now widely accepted that atherosclerosis is a chronic inflammatory process, and the lesion mainly consists of macrophages, lymphocytes, smooth muscle cells and extracellular matrix containing lipid (24, 25). Since genistein has anti-atherosclerotic action, it led us to hypothesize the genistein also inhibits the inflammatory process in atherogenesis. We have questioned whether genistein effects on the composition of histiocytes and/or smooth muscle cells in the lesion, because these two cell types are main part in atherosclerosis. But there was no difference between genistein addition and no addition groups on the two cell types, at least in the composition. Several explanations would be possible for these results. First, genistein has no direct effect on the macrophages-smooth muscle cells in the inflammation. Second, the effects of genistein on these two cells were similar.
It has been suggested that one of the mechanisms by which estrogen replacement therapy may reduce the cardiovascular risk among postmenopausal women is the improving vascular reactivity (20-22). Since estrogen receptors are present in the blood vessel wall (26), genistein is able to act as estrogen agonist on the tissue (27). Some researches about phytoestrogen showed that it improved arterial stiffness (28) and potentiated endothelium-dependent vasodilatation (7). Honore et al. (29) reported that isoflavone enhances coronary vascular reactivity in atherosclerotic female monkeys.
Teede et al. (30) proposed that the reduction in arterial stiffness by estrogen or phytoestrogen may be an important factor in the apparent reduction in cardiovascular risk as demonstrated in epidemiological studies. A significant number of acute thrombotic occlusion that develops between angiograms occur in arterial segments that were angiographically normal or mildly irregular in the first angiogram (31). Practically, the acute, often unheralded, onset of symptoms in acute myocardial infarction suggests that pre-existing coronary stenoses susceptible to acute thrombotic occlusion in the infarct-related artery may not necessarily have been severe (32). In addition, the vulnerable plaques occur across the full spectrum of severity of stenosis, and disruption of a plaque causing minimal stenosis is more likely to invoke an acute ischemic episode because of the lack of prior collateral development (33). Rioufol et al. (34) suggested that the acute coronary syndrome is more connected with overall coronary instability rather than one single lesion when observed by their intravascuar ultrasound study. Therefore, the character of the atherosclerotic lesions may be more important than the size itself.
Aikawa et al. (35) demonstrated that lipid lowering by dietary manipulation significantly reduces proteolytic activity and increases collagen content of estabilished atheroma in rabbits, when MMP-1 activity was measured. Bocan et al. (36) showed that MMP expression was reduced by cholesterol esterification enzyme inhibitor. Their results suggested that lipid lowering may stabilize vulnerable plaques by reduced activity of the enzymes that degrade the arterial extracellular matrix, and render atheroma less susceptible to disruption and thrombosis by favoring collagen accumulation in the fibrous cap. In fact, with atherosclerotic lesion development, the expression of matrix metalloproteinase-1, -3, -7, and 9 increase (37, 38).
In this experiment, we could see decreased MMP-3 expression in the aorta in genistein supplementmentation and lipid lowering rabbits. The decreased expression of the enzyme may contribute to stabilization of the lesion, since the enzyme involved in plaque rupture in the advanced lesion.
We conclude that genistein, a type of phytoestrogen, inhibits aortic atherosclerosis initiated by hyperlipidemic diet in rabbits. The mechanism for this lesion inhibition might be due to decrease endothelial dysfunction by inhibiting oxidative modification of LDL cholesterol, rather than direct action on macrophage or smooth muscle cells in the atherosclerotic lesion.
The results also suggest that genistein may represent a good candidate to substitute estrogens in the prevention of atherosclerosis for stabilization of the atherosclerotic lesion. We think further studies are needed for understanding of mechanism of anti-atherogenic action of phytoestrogen.
Figures and Tables
 | Fig. 1Aortic lesion development in rabbit aortas (Mean±SDs). Genistein supplementation showed reduction of the lesion size. ND, normal diet; HD, hypercholesterol diet; G, genistein; TA, thoracic aorta; AA, abdominal aorta. *p<0.05. |
 | Fig. 2Anti-RAM-11 immunohistochemical staining. There are strong staining of anti-RAM-11 in the superficial and deep areas of the atherosclerotic lesion. HD+G group (A), ND group (B) (×40). |
 | Fig. 3Anti-HHF-35 immunohistochemical staining. Mild staining of anti-HHF-35 mainly in the deep areas of the atherosclerotic lesion. HD+G group (A), ND group (B) (×40). |
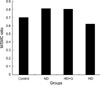 | Fig. 4The macrophage (M)/smooth muscle cell (SMC) ratio in the atherosclerotic lesion in rabbit aortas. ND, normal diet; HD, hypercholesterol diet; G, genistein. |
References
1. Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl. 1990. 201:3–23.

2. Wong WW, Smith EO, Stuff JE, Hachey DL, Heird WC, Powell HJ. Cholesterol-lowering effect of soy protein in nomocholesterolemic and hypercholesterolemic men. Am J Clin Nutr. 1988. 68:1385S–1389S.
3. Lichtenstein A. Soy protein, isoflavones and cardiovascular disease risk. J Nutr. 1998. 128:1589–1592.

4. Lee BS, Won JW, Lee SK, Choi Y, Yoon S, Park KH, Cho DJ, Song CH. The effect of isoflavone on serum lipid profiles and bone markers in postmenopausal woman. J Korean Soc Meno. 2002. 8:59–67.
5. Dubey RK, Gillespie DG, Imthurn B, Rosselii M, Jackson E, Keller P. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999. 33:177–182.

6. Nestel PJ, Pomeroy SE, Sasahara T, Yamashita T, Liang YL, Dart AM, Jennings GL, Abbey M, Cameron JD. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flasxseed oil despite increased LDL oxidizability. Arterioscler Thromb Vasc Biol. 1997. 17:1163–1170.
7. Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17β-estradiol. Circulation. 2001. 103:258–262.

8. Murkies A. Phytoestrogens-what is the current knowledge? Australian Family Physician. 1998. 27:Suppl 1. S47–S51.
9. Yamakoshi J, Piskula MK, Izumi T, Tobe K, Saito M, Kataoka S, Obata A, Kikuchi M. Isoflavone aglycone-rich extract without soy protein attenuates atherosclerosis development in cholesterol-fed rabbits. J Nutr. 2000. 130:1887–1893.

10. Libby P, Aikawa M. New insights into plaque stabilization by lipid lowering. Drugs. 1998. 56:Suppl. 9–13.
11. Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989. 135:169–175.
12. Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991. 11:1223–1230.

13. Hansson GK, Jonasson L, Holm J, Clowes MM, Clowes AW. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and vitro. Circ Res. 1988. 63:712–719.
14. Galis ZS, Sukhova GK, Libby P. Microscopic localization of active proteases by situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995. 9:974–980.
15. Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques: potentioal role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995. 92:1565–1569.
16. Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinkerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998. 97:2433–2444.
17. Shiomi M, Koh T, Tsudaka T. Reduction of serum cholesterol levels alters lesional composition of atherosclerotic plaques: effect of pravastatin sodium on atherosclerotis in mature WHHL rabbits. Arterioscl Thromb Vasc Biol. 1995. 15:1938–1944.
18. Carson FL, Coleman SA, Futch HN. Hrapchak BB, Sheehan DC, editors. Connective tissue and muscle fiber stains. Theory and practice of histotechnology. 1987. Columbus, HO: Battelle Press;196–197.
19. Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ohman L, Greene Gl, Gustafsson JA, Carlquiest M. Molecular basis of agonism and atntagonism in the oestrogen receptor. Nature. 1997. 389:753–758.
20. Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990. 81:1680–1687.

21. Gilligan DM, Quyyumi AA, Cannon RO III. Effects of physiological levels of estrogen on coronary vasomotor function in post-menopausal women. Circulation. 1994. 89:2545–2551.

22. Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P. Estrogen improves endothelium-dependent, flow mediated vasodilation in postmenopausal women. Ann Intern Med. 1994. 121:936–941.
23. Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, Deodato B, Ferlito M, Campo GM, Bova A, Caputi AP. Genistein supplementmentation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovascular Research. 2000. 45:454–462.
24. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993. 362:801–809.

25. Ross R. Mechanism of disease: Atherosclerosis - An inflammatory diseasee. N Engl J Med. 1999. 340:115–126.
26. Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic. coronary arteries of postmenopausal women. Circulation. 1994. 89:1501–1510.
27. Stahl S, Chun T, Gray WG. Phytoestrogens act as estrogen agonists in an estrogen-responsive pituitary cell line. Toxicol Appl Pharmacol. 1998. 152:41–48.

28. van der Schouw YT, Pijpe A, Lebrun CE, Bots ML, Peeters PH, van Staveren WA, Lamberts SW, Grobbee DE. Higher usual dietary intake of phytoestrogens is associated with lower aortic stiffness in postmenopausal women. Arterioscler Thromb Vasc Biol. 2002. 22:1316–1322.

29. Honore EK, Williams JK, Anthony MS, Clarkson TB. Soy isoflavones enhance coronary vascular reactivity in atherosclerotic female macaques. Fertil Steril. 1997. 67:148–154.
30. Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness A placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003. 23:1066–1071.
31. Haft JI, Haik BJ, Goldstein JE, Brodyn NE. Development of significant coronary artery lesions in areas of minimal disease: a common mechanism for coronary disease progression. Chest. 1988. 94:731–736.
32. Hackett D, Davies G, Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988. 9:1317–1323.

33. Mann JM, Davies MJ. Vulnerable Plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation. 1996. 94:928–931.
34. Rioufol G, Finet G, Ginon I, André-Fouët X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF, Tabib A. Multiple Atherosclerotic Plaque Rupture in Acute Coronary Syndrome. Circulation. 2002. 106:804–808.

35. Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma. Circulation. 1998. 97:2433–2444.

36. Bocan TM, Krause BR, Rosebury WS, Mueller SB, Lu X, Dagle C, Major R, Lathia C, Lee H. The ACAT inhibitor Avasimibe reduces macrophages and matrix metalloprotenase expression in atherosclerotic lesions of hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 2000. 20:70–79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download