Abstract
As the treatment of chronic non-cancer pain gradually increases, clinicians have more opportunities to encounter opioid prescription. However, guidelines for prescribing opioids for chronic non-cancer pain have never been published in Korea. The present guidelines were prepared by reviewing various research data. In cases in which the data were insufficient, recommendations were presented following discussion among experts affiliated with the Opioids Research Group in the Korean Pain Society. The present guidelines may need to be continuously revised and amended as more clinical evidence is acquired.
Guidelines for prescribing opioids for chronic non-cancer pain have been published in the U.S., Europe, and Japan, but not in Korea.
The present prescription guidelines do not provide absolute prescription standards, but guide the doctors in charge of pain treatment to prescribe opioids to patients with chronic non-cancer pain on the basis of currently available scientific evidence. The present guidelines were prepared by reviewing various research data published in Korea and other countries as well as issues involved in the prescription of opioids in Korea. In cases in which the data were insufficient, recommendations were presented following discussion among experts affiliated with the Opioids Research Group in the Korean Pain Society. Therefore, final decisions regarding prescription to individual patients should be made by doctors taking care of the patients, considering various situations. The present prescription guidelines should neither be used to limit the prescription of opioids by doctors nor as a basis for reviewing health insurance or for making judicial decisions with respect to the prescription of opioids to a specific patient.
The present guidelines are expected to assist in the prevention of problems caused by excessive, inappropriate, and delayed prescription of opioids, as well as to reduce confusion in the communication between clinicians who care for patients and researchers. Since the data regarding the prescription of opioids in Korea are currently insufficient, the present guidelines do not reflect the data to any significant extent, but a future revised version of the present guidelines will reflect more domestic data. Since these guidelines are the first guidelines to be published in Korea, the current version is to be corrected or amended if there were to be new academic findings.
Also, partial agonist/antagonists were excluded from the present guidelines, and Tramadol, Ultracet, and Codeine were classified as opioid analogues.
The causes and characteristics of pain should be investigated through detailed history taking and physical examination. The investigation should include the history of interventional procedures for pain and the medication history including recent administration of opioids. Specific situations in which pain is relieved or exacerbated should also be investigated. In addition to the Numeric Rating Scale (NRS) and the Visual Analog Scale (VAS), various assessment tools shown below may be used to measure functional status and pain intensity (Table 1) [1].
Patients who have psychic or psychological problems are more likely to abuse opioids than those who do not have these problems. Therefore, before prescribing opioids, the presence of depression, anxiety, and somatization should be assessed. In cases of personality disorders, an additional cooperative diagnosis with the department of neuropsychiatry is required.
Sleep is an important factor in maintaining body functions. Especially when systemic pain such as fibromyalgia is present or to people of advanced age, the maintaining of normal sleep is critical.
History of smoking, alcohol, and substance abuse should be investigated. It is also critical to investigate the history of abuse in the past.
No guidelines consider opioids as a first-line treatment. Opioids may be considered as a second-line or third-line treatment method when all other treatment methods, such as physical therapy, exercise therapy, and interventional procedures have been used on pain that is of a moderate or higher level, or which appears to have an organic factor, but the results have been unsatisfactory. In addition, a thorough assessment that clarifies the presence of a psychic or psychological disorder should be done in order to differentiate psychogenic pain from other types of pain.
Before initiating opioid administration, the lesions or diseases causing pain should be identified as clearly as possible, and nonspecific diagnoses such as back pain and knee joint pain should be avoided.
Obtaining the history of a patient's drug administration is critical before prescribing opioids and a prescription search system is useful in this regard. Studies have shown that use of such a prescription search system reduces drug abuse and doctor shopping and as of 2012, a prescription search system is available in 38 states in the United States. As the use of electronic prescriptions has recently become extensive in Korea, access to a patient's history of drug administration has become more convenient. Establishment of a national integrated information network could be an urgent project.
Many treatment guidelines with regard to the prescription of opioids recommend urine drug testing (UDT) before the start of opioid prescription to reduce the risks related to the administration of these agents [3]. Accurate understanding of pharmacology, pharmacokinetics, and drug interactions is important for appropriate interpretation of the test results (Table 3).
In order to prevent damage to the doctor-patient relationship, a policy that requires the implementation of UDT for all patients initiating opioid administration may be established. Such policy may prevent an unnecessary stigma effect, but the implementation is practically difficult in Korea. Alternatively, UDT may be mandated in a restrictive fashion to cases in which addiction or abuse of opioids is strongly suspected.
The Guidelines published by the Japan Society of Pain Clinicians recommend initiating opioid administration after providing the following information to patients who have non-cancer pain [4].
(1) The initiation, dose control, and discontinuation of opioid administration are determined by the doctors.
(2) The ultimate goal of opioid treatment is to improve quality of life.
(3) The purpose of opioid treatment should be clearly described.
(4) The patient should have a clear understanding of the purpose of opioid treatment.
(5) The patient should receive the regular treatment determined by the doctor during the opioid treatment.
(6) The patient is not allowed to receive opioid administration from two or more medical institutions.
(7) Various side effects may be caused by long-term administration of opioids.
(8) Administration of opioids is not a life-long treatment.
(9) Opioids should never be given to another person.
(10) The formulation and the administration method should not be changed.
(11) When the opioid treatment is discontinued or the prescription is changed, the unneeded opioids should be returned to the doctor (or medical institution) as soon as possible.
In addition, receiving a written consent form, shown in the example of Fig. 1, is recommended to improve future therapeutic compliance, but the basis of receiving the consent is still limited.
The effect of treatment may be assessed by measuring the pain intensity using the NRS or VAS, or by performing a functional test using the Oswestry Disability Index or Neck Disability Index. Complete improvement of pain in patients with chronic non-cancer pain is very rare. Therefore, an appropriately recommended objective is to decrease the pain intensity by 30% or more, or to improve function by 30% or more.
While codeine and codeine and tramadol complexes are not classified as opioids in Korea, they are considered opioids in the guidelines of other countries. Therefore, they are classified as opioid analogues in the present guidelines.
Oral administration is the most convenient and economical method. A less invasive method may need to be considered if oral administration is impossible due to dysphagia or uncontrollable vomiting.
Rectal administration may be performed if the patient has nausea or vomiting, or is in a fasting state preoperatively or postoperatively. The dose for rectal administration is equal to that of oral administration.
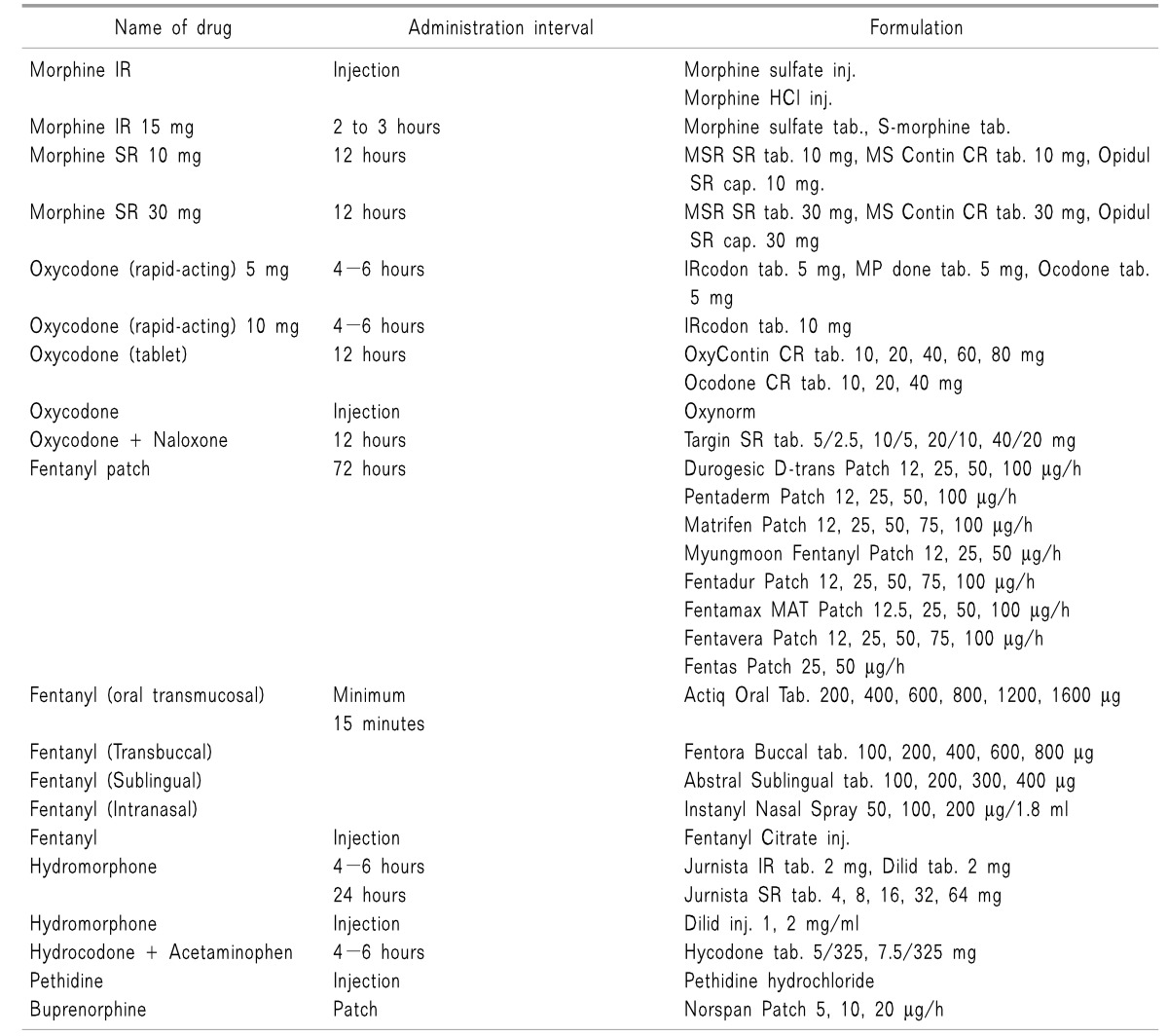
The fentanyl patch was the only medication for transdermal administration in the past, but the Norspan patch has recently become commercially available. The maximum dose of a fentanyl patch is 300 mg/h, and another method of administration needs to be considered in cases in which a higher dose is required.
Fentanyl is the only medication for oral mucosal administration. Oral mucosal administration is performed by rubbing the medication on the oral mucosa or by placing it under the tongue. The medication should be carefully administered to avoid swallowing. The onset of action is 5 to 15 minutes, and the maximum effect is reached in 20 to 30 minutes after the administration. The effect is maintained for about two hours.
Intramuscular injection is not recommended because it may cause pain and the absorption is uncertain.
Intravenous injection has several advantages. Intravenous injection may be performed when oral administration is impossible, a large number of tablets is required to meet the high dose, or the dose needs be increased rapidly. Morphine, hydromorphone, fentanyl, sufentanil, and oxycodone may be administered through intravenous injection.
Similarly to intravenous injection, hypodermic injection is performed when oral administration is impossible or continuous injection is required. When an instrument for continuous injection is used, the lockout time should be longer than that of intravenous injection, because the maximum plasma concentration is reached slowly in hypodermal injection.
The concept of opioid treatment differs completely between chronic non-cancer pain and cancer pain. In the treatment of cancer pain, the dose of opioid analgesic is increased in a short period of time until the pain is relieved. However, in the treatment of non-cancer pain, non-opioid analgesics are used initially, before applying an opioid analgesic [5]. If the pain is not controlled by non-opioid analgesics, opioid treatment is considered, starting from the minimum dose at which adverse effects may be tolerated. The dose is slowly increased over a sufficiently long observation period, even if the effect is verified at the early stage of the administration. This is because the acute onset of tolerance due to a rapid dose increase as well as dependence caused by the tolerance should be avoided.
Initially, the type of opioid and the dose should be determined on an individual basis considering the environment, accompanying diseases, history of opioid medication, purpose of the treatment, medical adverse effects, and degree of pain of each patient [6]. Prescription of opioids typically starts with a short-acting opioid, following a test period [7]. The test period is usually five weeks. The prescription starts with a low dose, which is then slowly increased to reduce adverse effects. Alcohol and other tranquilizers should be avoided during the process of determining the opioid dosage.
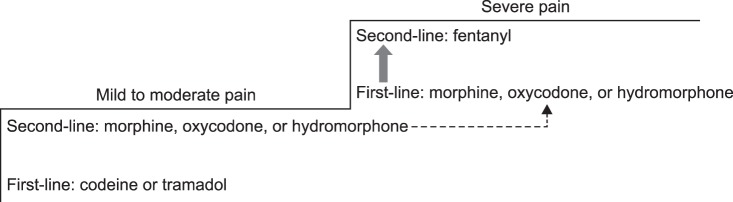
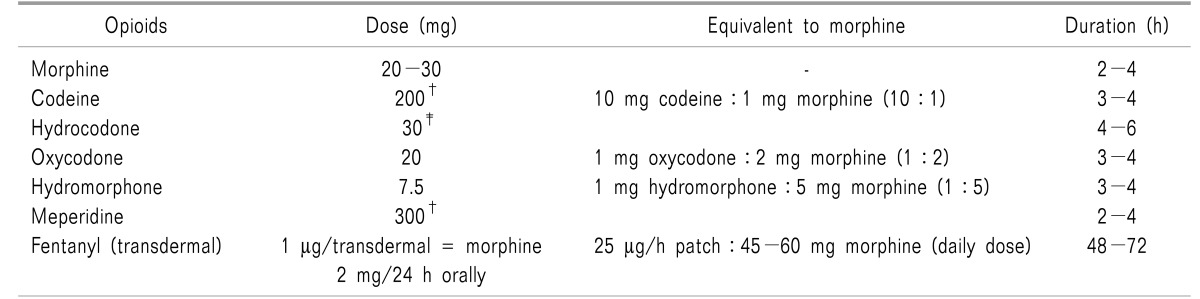
Initially, opioid analogues, such as codeine and tramadol, should be prescribed. An analgesic complex, including tramadol and a non-opioid analgesic, may also be prescribed. Codeine has an analgesic effect through its metabolite, morphine. Therefore, the same level of care required for morphine administration should be paid to the administration of codeine. The potency of codeine is 1/6 that of morphine. Codeine is metabolized by a drug metabolic enzyme in the liver, CYP2D6, and the analgesic effect of codeine is thereby weaker in patients with low CYP2D6 activity. Some Koreans (about 6%) have a low level of CYP2D6 activity [8].
A tramadol-acetaminophen complex tablet, which is classified as an opioid analogue, has an analgesic effect through the action of the µ-opioid receptors, the activation of the inhibitory system through the inhibition of noradrenalin-serotonin reuptake, and the action of acetaminophen. The risks of dependence and abuse are low, and drug withdrawal symptoms are known to be mild. Tramadol's own affinity for the opioid receptors is weak, but the affinity of M1, the metabolite, is strong. Since tramadol is also metabolized by the liver enzyme CYP2D6, the analgesic effect is weak in patients with low CYP2D6 activity [9].
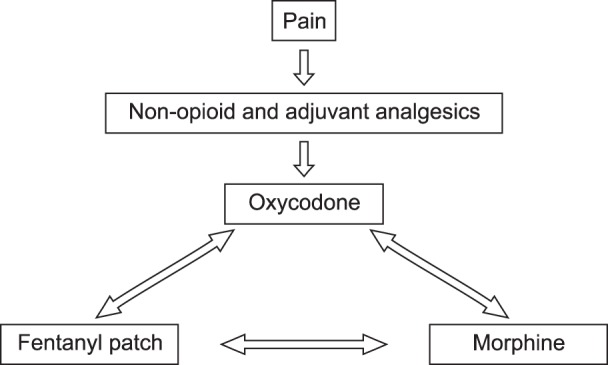
Use of opioids may be considered from the beginning for patients experiencing severe pain. Considering the ratio of effect to cost, morphine is an appropriate opioid medication, but oxycodone and hydromorphone may be used as alternatives (Fig. 2) [10].
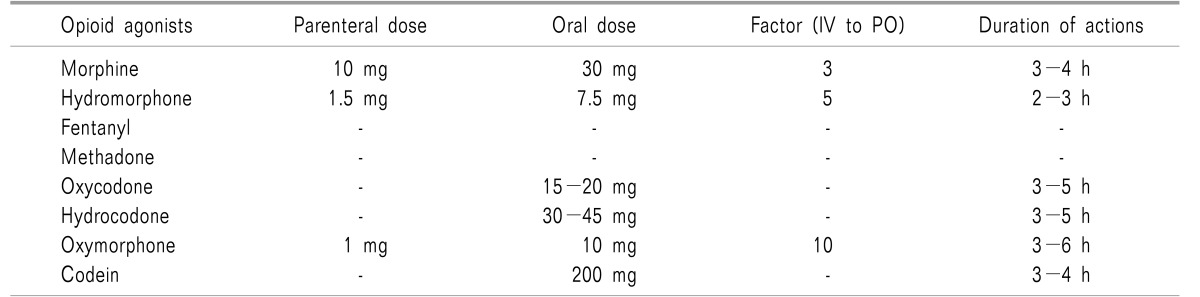
Morphine is classified as a strong opioid and the short-term or long-term adverse effects of morphine administration should be taken into account. Oxycodone, which is a strong opioid that is structurally analogous to morphine, may be used as an alternative to morphine, and the potency is 1/2 that of morphine. Hydromorphone is a strong opioid synthesized from morphine, and the potency of hydromorphone is two or three times that of oxycodone and five to seven times that of morphine [7]. Hydromorphone is relatively safe because a small dose can provide a strong analgesic effect without an active metabolite.
Pure agonists are known to have no analgesic ceiling effect. An exception is meperidine, but meperidine is not recommended for the control of chronic pain because of the risks of anxiety, agitation, and convulsions caused by the stimulation of the central nervous system due to normeperidine, an activation product of meperidine.
Combination agonists or mixed agonist/antagonists are not recommended [11].
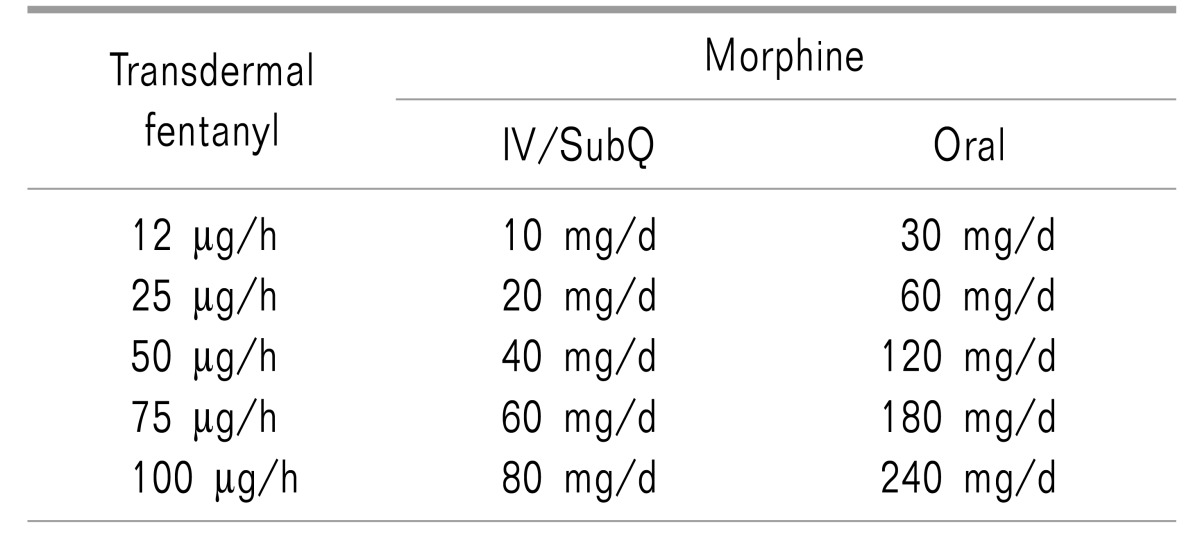
Use of a fentanyl patch from the early stage is not generally recommended for fear of an overdose. In exceptional cases, when oral administration is not possible due to poor nutrition or vomiting, use of a fentanyl patch is permitted. However, the use of a fentanyl patch should be limited to patients who were administered 60 mg or more of oral morphine equivalent per day for at least two weeks [7].
The initial opioid dose is dependent on each patient's health status, conventional medications, accomplishment of treatment goals, and predicted adverse effects. Administration of opioids should begin with the minimum dose that demonstrates an effect, and the dose may be gradually increased. Initially, using tramadol or a tramadol/acetaminophen mixture, classified as an opioid analogue, 50 mg of tramadol is administered four times a day in an administration interval of four hours or longer, not exceeding a maximum daily dose of 400 mg [7]. The dose of codeine is 20 mg for oral administration, and the maximum dose per day is 60 mg, which may be varied in consideration of patient symptoms and age. The initial dose of oral morphine administration is 5 to 10 mg in an interval of 4 to 6 hours, not exceeding a daily maximum dose of 20 mg. The dose increment should be 5 to 10 mg per day [12]. Intravenous injection of morphine starts with 2 to 5 mg of morphine or an equivalent opioid. Table 6 shows the equivalent opioids with reference to morphine dose. Sudden pain may be appropriately controlled using 10 to 20% of a total daily dose. If sudden pain occurs three or more times per day, the dose should be increased to a level of continuous medication [7]. Even though the effect may be insufficient in the early stage of treatment, a sufficiently long period of time should be allowed to observe the effect.
The initial fentanyl patch dose should be determined with reference to the dose and administration methods of previously used opioids. A buprenorphine patch (Norspan patch) containing 5 µg of buprenorphine may be used and replaced by a new patch every seven days. The appropriate dose may be increased or decreased depending on the analgesic effect and the symptoms of the patient, not exceeding a daily maximum dose of 20 µg.
The period of initial treatment may be several weeks to several months. However, if the symptoms are not improved but increased, a dose increase may be considered. Treatment continuation should be determined by verifying not only the relief of the pain but also the improvement in the patient's quality of life. At least one adverse effect is experienced by 80% of patients to whom opioids are administered. Hence, the presence of adverse effects, such as constipation, symptoms of addiction, and accompanying internal diseases, should be considered in determining the period of initial treatment.
A non-opioid analgesic should be prescribed to patients whose pain continues despite the opioid administration. If pain is not adequately improved by the administration of a specific opioid over a certain period of time, another opioid may be added in cooperation with a pain specialist. If satisfactory pain control is not achieved despite a sufficient increase in the opioid dose, ketamine may be used in combination with the opioid. Corticosteroid may be carefully used in combination with an opioid for certain causes of pain [6].
Tramadol should not be used in patients with a history of convulsions, because tramadol may increase the risk of convulsions when used in combination with a tricyclic antidepressant, a selective serotonin reuptake inhibitor (SSRI), or a monoamine oxidase (MAO) inhibitor [11]. Benzodiazepines may be selected according to a patient's state and need, but the initial administration of benzodiazepines should be performed after a prior dose decrease or discontinuation of an opioid analgesic, because benzodiazepines reduce the lethal dose of an opioid analgesic [12].
An observation period of one to four weeks should be allowed during initiation of opioid administration or dose increases, to permit assessment of the benefits and risks. In patients who require long-term treatment, continuation of opioid treatment should be reviewed every one or two months or even earlier. The dose should be reduced as much as possible or the administration should be discontinued through discussion with the patient.
In the prescription of opioid analgesics for chronic non-cancer pain, modification of the dose is not appropriate except in cases when the pain has become more severe due to changes in the pattern of the pain. If the dose of an opioid analgesic is increased only because a patient complains of pain, the patient may be confronted with excessive administration or problems associated with abuse and dependence. Patients may perform the activities of daily living with an appropriate opioid dose established for each patient. The prescription may be maintained at a fixed dose over a long period of time, the dose may be gradually decreased, or the administration may be appropriately discontinued.
The Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Non-cancer Pain published by The American Pain Society and the American Academy of Pain Medicine state that the risk of abuse and dependence is greater than the benefit of treatment in cases in which 200 mg or more morphine milligram equivalent (MME) per day is administered, and thus the opioid treatment should be reconsidered [13].
The Guidelines for the Prescribing Opioid Analgesics for Chronic Non-cancer Pain published by the Japan Society of Pain Clinicians established the maximum daily dose as 120 mg MME [4]. The recent standard published by the US Centers for Disease Control and Prevention (CDC) is stricter, at a maximum daily dose of 90 mg MME [14].
If neuropathic pain, represented by postherpetic neuralgia, diabetic nephropathy, etc., and complex regional pain syndrome are not well controlled using anticonvulsants or antidepressants, opioid administration may be considered as a second-line or third-line medication. The analgesic effects of opioids are known to be almost equal to the analgesic effects that may be obtained from other drugs used for neuropathic pain [15]. For patients initially administered opioids, oral administration of 5 to 15 mg short-acting morphine, or an analgesic equivalent of other short-acting opioids given by oral administration or injection, may be used, and the formulation may be changed to tablets after the maximum daily dose is approximately determined. The degree of pain control and the occurrence of adverse effects should be regularly assessed through consultation with patients.
In chronic osteoarthritis at knee joints or hip joints and myofascial pain syndrome, if patients do not respond to treatment with conventional non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, limited use of opioids may be considered. Administration of a single opioid is not recommended, but combined treatment of opioids with conventionally used drugs is recommended.
Partial use of opioids may be considered if pain at a body part on which surgery has been performed is not controlled with a treatment method other than opioid administration for two months or longer following a surgery such as thoracotomy, hernia surgery, amputation, mastectomy, or open heart surgery.
Short-acting or long-acting opioids have been known to be effective in pain control in patients with low back pain that is not controlled with conventional medications [16]. Combined treatment with another drug, rather than independent opioid administration, is helpful to control pain that may occur during rehabilitation or behavioral therapy.
Use of opioids in chronic neck pain has been poorly studied. One report showed that short-term administration of 5 to 10 mg oxycodone was effective in the control of acute neck pain [17]. It was also reported that intravenous injection of 0.3 mg/kg morphine in patients with chronic whiplash injury decreased the pain by 50% or more [18]. Therefore, limited use of opioids may be considered if chronic neck pain is not well controlled with the administration of non-opioid medications.
When the first-line treatment is ineffective in chronic headaches or when there are contraindications to the first-line treatment, opioids may be considered as an adjuvant analgesic. Use of opioids is not recommended in trigeminal neuralgia or atypical orofascial pain.
The assessment of patient opioid use, the reasons for opioid prescription, the overall pain control plan, medical requests, and periodical assessments of patient condition should be documented for the patient's records [19].
The degree of the analgesic or adverse effects of opioids used in clinical settings is dependent on individual patients and the medications used. Based on this fact, a currently used type of opioid may be replaced by another type to improve the quality of pain control by improving the analgesic effects and reducing the adverse effects. This replacement is called opioid rotation (Fig. 3). In the following cases, opioid rotation should be considered.
1. An adverse effect is not reduced even when sufficient countermeasures have been taken against the adverse effect.
2. No sufficient analgesic effect is obtained despite appropriate increments of dose.
3. Drowsiness or neurotoxicity occurs due to decreased renal function.
4. Administration route may not be changed if the same drug is used.
For opioid rotation, the total amount of opioid currently administered should first be calculated, and the equianalgesic dose of the drug that will be substituted should then be calculated. Administration of the new drug should begin at 50-75% of the calculated equianalgesic dose. The new opioid may be appropriately titrated by observing the adverse effects and the degree of analgesia (The equianalgesic dose may be calculated by referring to Tables 7 and 8, or by using the “Cancer Pain Control” app provided by the Ministry of Health and Welfare and the National Cancer Center).
For example, for a 62-year-old male patient who is administered 20 mg morphine twice a day for chronic pelvic pain, to replace morphine with hydromorphone, the equianalgesic dose is hydromorphone 4 mg per day because the ratio of oral hydromorphone to oral morphine is 1 to 5. Assuming that the administration begins with 75% of the calculated equianalgesic dose, a total of 3 mg of hydromorphone may be administered separately, and the dose may be titrated by observing appropriate analgesic and adverse effects.
Regular verification of medication is essential for all patients receiving opioids. Attention should be paid to opioid administration because patients are often administered many drugs, and an adverse effect may thereby be caused through the interaction between drugs [20]. In control of the dose of a drug, it is necessary to determine the minimum dose that may minimize adverse effects and provide the optimal effect.
A patient's medication history must be verified at every visit, including the addition of a new drug or the replacement of currently administered drugs. The history of benzodiazepines, over-the-counter sedatives, and alcohol intake should be verified as well as the weekly total of the drugs administered to a patient [21]. The dose should be determined every week when initiating opioid administration, or when the dose is increased. The interval between patient visits may be long if the patient has a low risk of drug abuse or small dose fluctuation. However, performing appropriate medical examination and/or follow-up are usually recommended within one or two months. Patients who have a high risk of aberrant drug-related behaviors, or who require frequent dose adjustments, should have shorter intervals between visits. Aberrant drug-related behaviors, analgesia, adverse effects, and the activities of daily living should be described at every visit.
Since it was reported that oral opioids are effective in patients with chronic non-cancer pain without a history of mental illness or substance use disorder, and that the possibility of addiction is low [22], the administration of opioids to patients with chronic pain has drastically increased [2324]. As a result, causalities and hospital visits due to the adverse effects of excessive opioid intake have also increased [25].
The risk of excessive administration is 1.6 to 4.6 times higher in patients who are administered 50-100 mg MME per day than in patients who are administered 20 mg MME per day or less [2627], indicating that the risk of excessive administration is increased as the dose is increased. Therefore, the maximum dose of opioids in patients with chronic non-cancer pain must be limited. At any dose, patients should be advised of the risk of excessive administration at the time of initial opioid administration. When the dose is increased to 50 mg MME per day or higher, the risks and benefits of the increased dose to the patient should be re-evaluated. When the dose is increased to 90 mg MME per day or higher, dose control should be carefully performed [14]. Most studies have shown that the dose is controlled within a range of 180 mg MME per day in cases of non-cancer pain [28]. If the dose is frequently or drastically increased, incorrect medication or drug abuse should be suspected despite improvement in pain, and more frequent assessment of administration, non-drug treatment, and multi-department treatment are recommended.
When a patient is administered benzodiazepines, simultaneous prescription of an opioid should be avoided to prevent central nervous system depression. Discontinuation of benzodiazepines should be performed gradually to prevent withdrawal symptoms. To resolve a patient's anxiety, psychiatric treatment or another drug should be used.
If there is a severe adverse effect or if pain is not reduced despite an increase of the dose, another opioid analgesic may be tried. Differences in metabolic activity due to genetic polymorphisms of cytochrome P may decrease the effect of opioids or increase adverse effects. In addition, no cross-tolerance of opioids has been reported [2930].
The Opioids Research Group in the Korean Pain Society provides a checklist for opioid prescription through revision of the guidelines published by the US CDC to reflect the situation in Korea (See the attached checklist).
Unless adverse effects of opioid-based treatment of non-cancer pain are properly managed, the effects of pain treatment may be significantly decreased as drug administration is discontinued. Therefore, appropriate counter-measures to the adverse effects are critical to the success of pain treatment. Preparing countermeasures in advance is recommended because adverse effects on the digestive system may occur even at appropriate doses.
When acute opioid overdose is suspected, naloxone should be administered after observing the vital signs, supplying oxygen, and securing intravenous access. For adults, 0.2 mg of naloxone is intravenously injected in general. If the effect is not sufficient, 0.2 mg of naloxone may be additionally injected after an interval of several minutes. When critical symptoms are observed, advanced cardiovascular life support (ACLS) should be performed.
Nausea and vomiting usually occur when opioid administration is initiated or the opioid dose is increased. Nausea and vomiting respond well to antiemetic drugs and improve in three or four days as tolerance develops. Nausea and vomiting may be found in many patients even before opioid administration because of metabolic disorders, anticancer treatment, radiotherapy, enterocleisis, and stomach ulcers. Metoclopramide or a 5-HT3 receptor antagonist (ondansetron), or a dopamine D2 receptor antagonist (domperidone) may be administered for treatment.
Constipation is predicted in almost all cases of opioid administration. Hence, treatment for constipation begins with the initiation of opioid treatment. A stool softener and a motor stimulant may be administered for the treatment of constipation caused by opioid administration. If a stool softener and a motor stimulant are not effective, lactulose is administered.
At the early stage of morphine administration or following dose increases, symptoms such as cognitive disorder, amnestic disorder, thought disorder, hallucinations, and drowsiness may occur in patients who are advanced in age or who have generalized weakness. Drowsiness is often found with dose increases or in patients who have not slept well due to the pain before opioid administration. Drowsiness is usually improved in two or three days in most patients as tolerance is developed. If drowsiness continues, methylphenidate or modafinil may be administered.
It has been reported that psychological dependence may occur in normal persons but rarely in patients with pain. Physical dependence may occur even in patients with pain, but the degree of physical dependence is negligible in comparison to opioid abuse. It has been reported that withdrawal symptoms may be prevented by reducing the dose.
Respiratory depression usually occurs through the action of opioids on the µ-opioid receptors. Direct action of opioids on the respiratory centers in the brain reduces the reactivity to carbon dioxide (CO2), resulting in respiratory depression. Since respiratory depression may be caused by an analgesic dose, excessive administration should be carefully prevented.
At an analgesic dose, subcutaneous blood vessels may be expanded and histamine may be secreted, resulting in sweating and skin pruritus. Histamine H1 receptor antagonists or a small amount of naloxone may be used to treat pruritus.
Recently reported symptoms of neurotoxicity caused by opioid administration include cognitive disorder, severe sedation, hallucinations, mental aberration, muscular convulsions, hyperpathia, and dysesthesia. These symptoms of neurotoxicity are usually found in patients who have undergone long-term, high-dose opioid administration or who have accompanying metabolic disorders or renal failure. Methods of treating neurotoxicity include medication changes, sufficient fluid supply, and dose decreases.
Use of opioids may lead to opioid use disorder. According to the definition of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) [31], opioid use disorder refers to the integration of opioid dependence and substance abuse, considering the diseases not as separate diseases but a single disease with symptoms of different severities.
Due to the extensive definition by DSM-5, an individual who satisfies only two of the criteria is considered to have mild opioid use disorder (Table 9). Since tolerance and withdrawal, which are naturally found in patients with long-term opioid use, are included in the diagnostic criteria for opioid use disorder, all patients under long-term opioid administration are considered to have opioid use disorder. Therefore, if opioids are properly used for pain treatment, tolerance and withdrawal should be excluded from the diagnostic criteria for opioid use disorder. Even if they are excluded from the criteria, the lifetime incidence is as high as 21% among patients under chronic opioid administration, according to the DSM-5 criteria for opioid use disorder [32].
Since opioid use disorder is often caused by a patient's incorrect use of the drugs, doctors should carefully observe patient awareness of accurate drug use methods. Close monitoring is necessary to ensure appropriate use of opioids and to prevent abuse. Drug intensity tests, regular outpatient visits, and urine drug tests may be helpful, and these methods have reduced drug abuse by about 50% [3334]. A screening questionnaire for opioid addiction may be used to identify patients who are vulnerable to addiction, but the reliability of currently available questionnaires is not high [35]. Urine drug tests may be performed for patients at high risk for drug addiction, but the method is not effective in Korea, considering the high cost, inaccuracy of the tests, and the difficulty of obtaining non-medical opioids in Korea.
Tolerance to opioids should be carefully monitored. If a patient requires more drugs to obtain the same level of analgesic effect, it should be verified whether or not the patient is taking the drugs according to the instructions and whether or not tolerance is also being developed due to use of another type of opioid. If necessary, the effect of opioid administration should be examined under monitoring by medical staff.
Opioid use disorder is difficult to diagnose. Most patients tend to hide symptoms or abnormal behavior relevant to opioid use disorder. In addition, abnormal behavior identified may be the result of pain that has not been sufficiently treated or a cognitive disorder. Therefore, abnormal behavior should be carefully monitored, and the assistance of an expert should be obtained [21], if necessary. Particularly when a patient is addicted to another drug, opioids should be prescribed after the addiction is treated or improved.
When unbearable adverse effects continue, the dose of opioid administration should be rapidly decreased. The main adverse effects of opioids, such as nausea, vomiting, constipation, and drowsiness, decrease a patient's quality of life and the activities of daily living.
Patients who are advanced in age may experience psychiatric symptoms, such as decreased cognitive function and delirium, during opioid treatment. Therefore, dose decrease should be attempted to avoid accidents, such as falls, through discussion with the patients and their families.
Discontinuation of opioid analgesic administration may cause withdrawal syndrome due to the physical dependence on opioids. Risk factors for withdrawal syndrome are high-dose administration and long-term use, but withdrawal syndrome may occur in patients under low-dose opioid administration.
More careful monitoring should be performed when the dose of opioids is decreased. Sufficient explanation should be provided to patients and their families.
Each week, the total opioid dose may be decreased by 10 to 50%. Rapid dose decrease within two to three weeks may be attempted when an adverse effect due to excessive administration is observed. The dose needs to be slowly decreased if a patient has been administered opioids over a long period of time, perhaps for several years. In such a case, the total opioid dose may be decreased by 10% each week or each month. Withdrawal of opioids in pregnant women may be related to sudden miscarriage or preterm birth.
Slow dose decrease is recommended so that opioid withdrawal symptoms (drug craving, anxiety, insomnia, abdominal pain, vomiting, diarrhea, diaphoresis, mydriasis, agitation, tachycardia, and piloerection) may be minimized. When initiating dose decrease, decreasing the total opioid dose by 10% each week may be established as a reasonable goal. Dose decrease should be performed considering a patient's condition. Once a dose decrease goal is achieved, dose decrease may be restarted after a short resting period. Once opioid administration is performed less than one time per day, discontinuation of opioid administration may be considered.
Ultra-rapid detoxication under anesthesia should not be attempted because it may cause death. A dose decrease plan for a pregnant woman should be prepared by considering the welfare of the mother and the fetus.
Patients may experience a craving for high-dose opioids or a suddenly return to the previous dose even after a successful dose decrease. Therefore, a non-opioid treatment should be performed in parallel, and emotional support should be provided to patients. If a patient shows signs of opioid dependence or addiction, an appropriate treatment or use of naloxone for preventing excessive opioid administration may be considered.
Steady monitoring of and discussion about possible adverse effects, and the possibility of discontinuing opioid administration, are necessary from the initiation of opioid administration. Discontinuation of opioid administration may be considered when the pain is found to be psychogenic pain, when pain is not sufficiently relieved, when an adverse effect is unbearable, when drug and treatment compliance is decreased due to abuse or dependence, when quality of life or the activities of daily living are impaired, and when the need for opioids has disappeared due to acceptance of pain. When abuse or dependence is suspected, a urine drug test may be performed at the doctor's discretion.
As the treatment of chronic non-cancer pain gradually increases in Korea, clinicians have more opportunities to encounter opioid prescription. The prescription of opioids for chronic non-cancer pain in Koreans is presumed to be different from that in Europeans and Americans, and thus prescription guidelines for Koreans are necessary. Therefore, the Opioids Research Group in the Korean Pain Society has prepared guidelines for prescribing opioids for chronic non-cancer pain for the first time in Korea. The present guidelines have been prepared to assist clinicians in prescribing opioids on the basis of currently available clinical evidence. The present guidelines may need to be continuously revised and amended as more clinical evidence is acquired through critical application of the guidelines by first-line clinicians and the reflection of up-to-date findings in Korea and other countries.
References
1. Paone D, Dowell D, Heller D. Preventing misuse of prescription opioid drugs. City Health Inf. 2011; 30:23–30.
2. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005; 6:432–442. PMID: 16336480.

3. Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2--guidance. Pain Physician. 2012; 15:S67–S116. PMID: 22786449.
4. The Committee for the Guidelines for Prescribing Opioid Analgesics for Chronic Non-cancer Pain of JSPC. Guidelines for prescribing opioid analgesics for chronic non-cancer pain. Tokyo: Shinko Trading Press;2012. p. 68–131.
5. Webster LR, Dove B. Avoiding opioid abuse while managing pain: a guide for practitioners. North Branch (MIN): Sunrise River Press;2007.
6. Yamaguchi T, Shima Y, Morita T, Hosoya M, Matoba M. Japanese Society of Palliative Medicine. Clinical guideline for pharmacological management of cancer pain: the Japanese Society of Palliative Medicine recommendations. Jpn J Clin Oncol. 2013; 43:896–909. PMID: 23885114.

8. Lee SY, Sohn KM, Ryu JY, Yoon YR, Shin JG, Kim JW. Sequence-based CYP2D6 genotyping in the Korean population. Ther Drug Monit. 2006; 28:382–387. PMID: 16778723.

9. Ko Y, Kim YH. The pharmacological management of neuropathic pain. J Korean Med Assoc. 2012; 55:582–592.

10. National Opioid Use Guideline Group (CA). Canadian guideline for safe and effective use of opioids for chronic non-cancer pain [Internet]. Ontario: Michael G. DeGroote National Pain Centre;2010. cited 2016 Oct 5. Available at http://nationalpaincentre.mcmaster.ca/opioid/.
11. Sansone RA, Sansone LA. Tramadol: seizures, serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009; 6:17–21.
12. Park HJ, Moon DE. Pharmacologic management of chronic pain. Korean J Pain. 2010; 23:99–108. PMID: 20556211.

13. Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009; 10:113–130. PMID: 19187889.

14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016; 315:1624–1645. PMID: 26977696.

15. Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA. 2005; 293:3043–3052. PMID: 15972567.

16. Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010; CD006605. PMID: 20091598.

17. Ma K, Jiang W, Zhou Q, Du DP. The efficacy of oxycodone for management of acute pain episodes in chronic neck pain patients. Int J Clin Pract. 2008; 62:241–247. PMID: 18070045.

18. Lemming D, Sörensen J, Graven-Nielsen T, Arendt-Nielsen L, Gerdle B. The responses to pharmacological challenges and experimental pain in patients with chronic whiplash-associated pain. Clin J Pain. 2005; 21:412–421. PMID: 16093747.

19. Lee WH. Use of opioid analgesics for the management of chronic noncancer pain. J Korean Pain Soc. 2004; 17(Suppl):S88–S93.

20. Geppetti P, Benemei S. Pain treatment with opioids: achieving the minimal effective and the minimal interacting dose. Clin Drug Investig. 2009; 29(Suppl 1):3–16.
21. Kahan M, Wilson L, Mailis-Gagnon A, Srivastava A. National Opioid Use Guideline Group. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 2: special populations. Can Fam Physician. 2011; 57:1269–1276. e419–e428. PMID: 22084456.
22. Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996; 347:143–147. PMID: 8544547.

23. Maxwell JC. The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev. 2011; 30:264–270. PMID: 21545556.

24. Cho YS, Lee JY, Kim HS, Kwon JH. Trends in the consumption of opioid analgesics in a tertiary care hospital from 2000 to 2012. Yakhak Hoeji. 2014; 58:268–276.
25. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers and other drugs among women--United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013; 62:537–542. PMID: 23820967.
26. Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011; 305:1315–1321. PMID: 21467284.

27. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011; 171:686–691. PMID: 21482846.

28. Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003; 349:1943–1953. PMID: 14614170.

29. Brennan MJ. The clinical implications of cytochrome p450 interactions with opioids and strategies for pain management. J Pain Symptom Manage. 2012; 44:S15–S22. PMID: 23218232.

30. Vissers KC, Besse K, Hans G, Devulder J, Morlion B. Opioid rotation in the management of chronic pain: where is the evidence? Pain Pract. 2010; 10:85–93. PMID: 20070552.

31. Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013; 170:834–851. PMID: 23903334.

32. Degenhardt L, Bruno R, Lintzeris N, Hall W, Nielsen S, Larance B, et al. Agreement between definitions of pharmaceutical opioid use disorders and dependence in people taking opioids for chronic non-cancer pain (POINT): a cohort study. Lancet Psychiatry. 2015; 2:314–322. PMID: 26360084.

33. Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011; 26:958–964. PMID: 21347877.

34. Manchikanti L, Manchukonda R, Damron KS, Brandon D, McManus CD, Cash K. Does adherence monitoring reduce controlled substance abuse in chronic pain patients? Pain Physician. 2006; 9:57–60. PMID: 16700282.
35. Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008; 24:497–508. PMID: 18574359.
Fig. 1
Example of a written consent form regarding the prescription of opioids for the treatment of chronic non-cancer pain [4].

Table 2
Example of a Screening Questionnaire to Assess the Possibility of Being Addicted to Opioids [2]
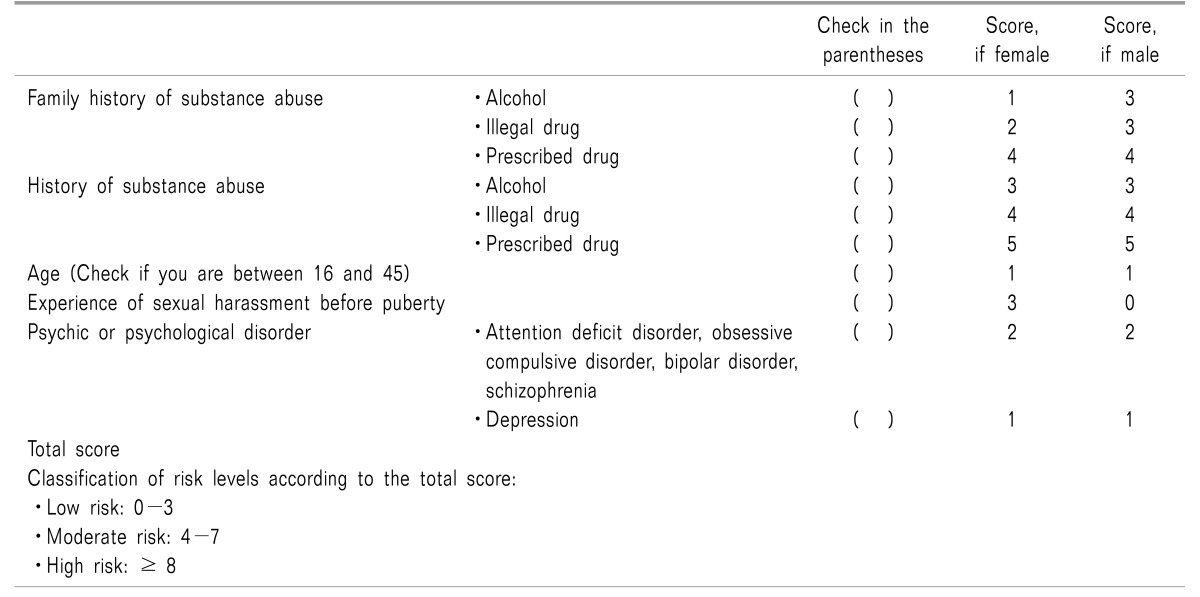
Table 3
Urine Drug Testing: Screening for Drug Abuse, Screening and Confirmation Cut-off Concentration, and Detection Time [3]




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download