Abstract
Background
Sacral nerve stimulation is a therapeutic option with demonstrated efficacy for conditions presenting with perineal pain caused by different etiologies. We aimed to assess whether a sacral electrode (InterStim®, Medtronic, Minneapolis, MN, USA) inserted through the caudal pathway is able to offer an acceptable level of sacral stimulation and rate of catheter migration.
Methods
We present 12 patients with pelvic pain who received sacral neuromodulation via the sacral hiatus with the InterStim electrode. We evaluated patient satisfaction as well as migration and removal of the electrode, if necessary.
Results
Our experience included 12 patients, 10 women and two men, with a mean age of 60 years. In eight of the 12 patients, the initial therapy was effective, and the final system implantation was performed. During subsequent follow-up, patient satisfaction was good. To date, there have been no cases of electrode displacement or migration.
Conclusions
The caudal insertion of the InterStim electrode, with its own fixation system, and initially designed for transsacral insertion, appears in our experience to be a satisfactory option which can minimize electrode displacements, achieving similar results in therapeutic efficacy and causing no difficulties in removal.
Sacral nerve stimulation is a therapeutic option with demonstrated efficacy for conditions presenting with perineal pain caused by different etiologies, such as interstitial cystitis, bladder dysfunction, vulvodynia, or post-radiation proctitis [123].
Although one of the traditional indications for sacral stimulation has been urological conditions [4567], for some years, other conditions such as coccydynias or certain proctological conditions have also been demonstrated to be appropriate for these therapies with satisfactory results [89101112].
The anatomical options for a sacral approach for nerve stimulation are: transsacral, retrograde, and anterograde (caudal).
Obviously, the anesthesiologist's experience will determine the choice regarding the method of approach; thus, the retrograde pathway, through lumbar epidural puncture and caudal advance of the catheter, appears to be an attractive pathway and, initially, a simple way to achieve sacral root stimulation. However, both in our experience as well as in the existing literature, there is a high incidence of an adequate advance of the electrode in the sacral promontory area being impossible, and therefore this approach shows a high rate of therapeutic failure [12313141516].
The transsacral approach is perhaps the least familiar to anesthesiologists, and therefore, it appears initially to be a more complex option, though there are a large number of publications regarding sacral root stimulation satisfactorily achieved with electrodes inserted through the S3 foramen [17181920].
Finally, the anterograde approach with caudal puncture (in the sacral hiatus) and cephalic advance of the electrode appears to be a simple and safe method of access, also allowing an easy and adequate advance of the electrode, enabling the stimulation of the entire sacral plexus [21].
These are the reasons why in recent years there have been many published cases in which this third option is the pathway chosen for sacral stimulation [20]; most articles agree that it results in the easy insertion and advance of the electrode, but there have been frequent reports regarding a relatively high incidence of electrode migration or later movement.
This article is the result of the interest in our unit regarding the possibility of implanting a transforaminal sacral electrode (InterStim®, Medtronic, Minneapolis, MN, USA) through a caudal approach, with the objective of achieving a lower rate of catheter migration, plus correct stimulation of the sacral plexus.
The InterStim electrode is a device designed to be inserted through the transsacral foramen pathway (through the S3 foramen), with the special feature of its own fixation system, which depends on the electrode.
The primary objective of our research was to assess whether an InterStim electrode inserted through the caudal pathway is able to offer an acceptable level of sacral stimulation and rate of catheter migration with easy insertion and removal, if necessary.
From October 2009 to April 2013, 12 sacral electrodes (InterStim) were implanted through the caudal pathway in our hospital unit.
The most frequent indication was chronic pain caused by coccydynia, followed by chronic urological dysfunction caused by interstitial cystitis.
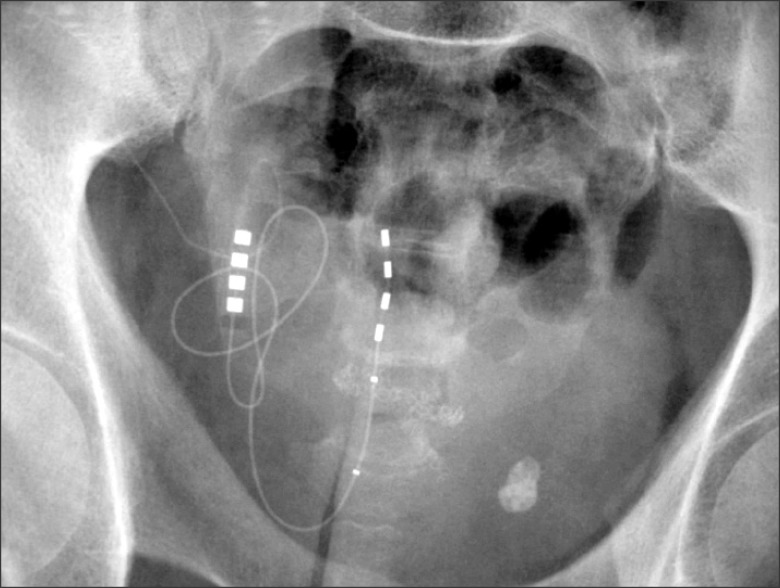
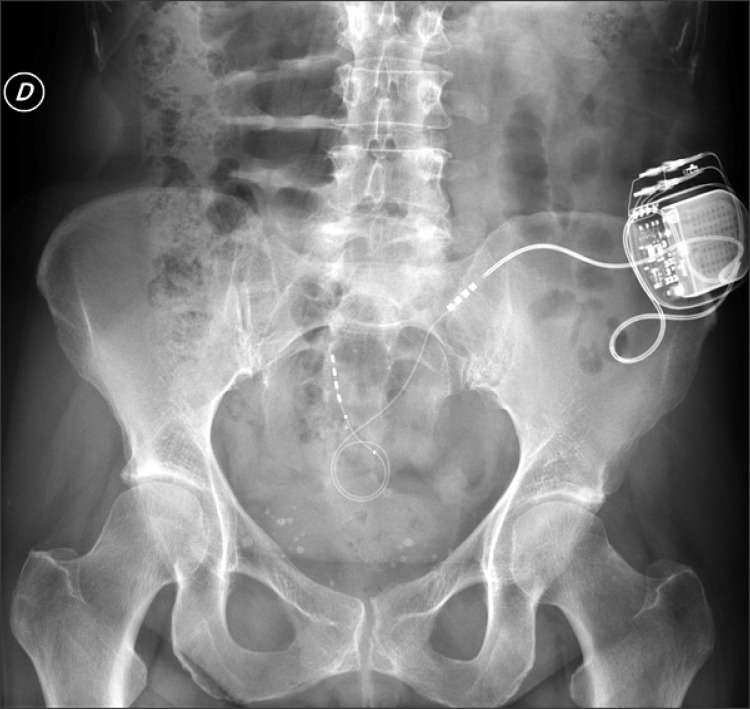
The electrode insertion was conducted percutaneously, under local anesthesia, and guided by radioscopy (Fig. 1 and 2).
The electrode is inserted percutaneously through the sacral hiatus in which we initially perform a puncture to reach the epidural space.
Then we channel the space with an introducer needle and glide the electrode to place it close to S3, and perform a stimulation test. If we obtain both positive motor and sensory responses in S3 (plantar flexion of the first toe and perineal paresthesia), we conclude that the electrode is in the optimal place and we remove the introducer, leaving the electrode in the definitive position.
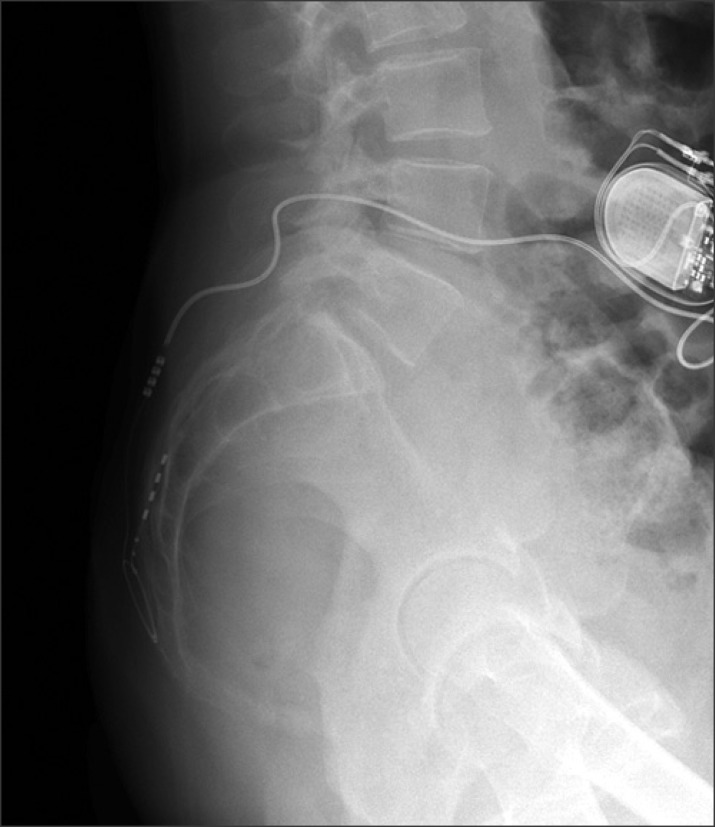
We connect the electrode to an extension line placed subcutaneously in the gluteal region. If the definitive system is placed, this connection is removed and replaced by a short extension line that binds to the pulse generator placed in the lower back region (Fig. 3).
Following electrode insertion, the patients underwent a testing stage, with telephone follow-up of their evolution, the therapeutic efficacy assessing the Subjective Improvement (SI) in pain, and the development of potential complications.
After this period, which lasted between fifteen and twenty days, the final system was implanted, or it was removed, according to the results.
In those patients with sacral stimulation implantation, follow-up visits were conducted at one month, three months, and every six months subsequently
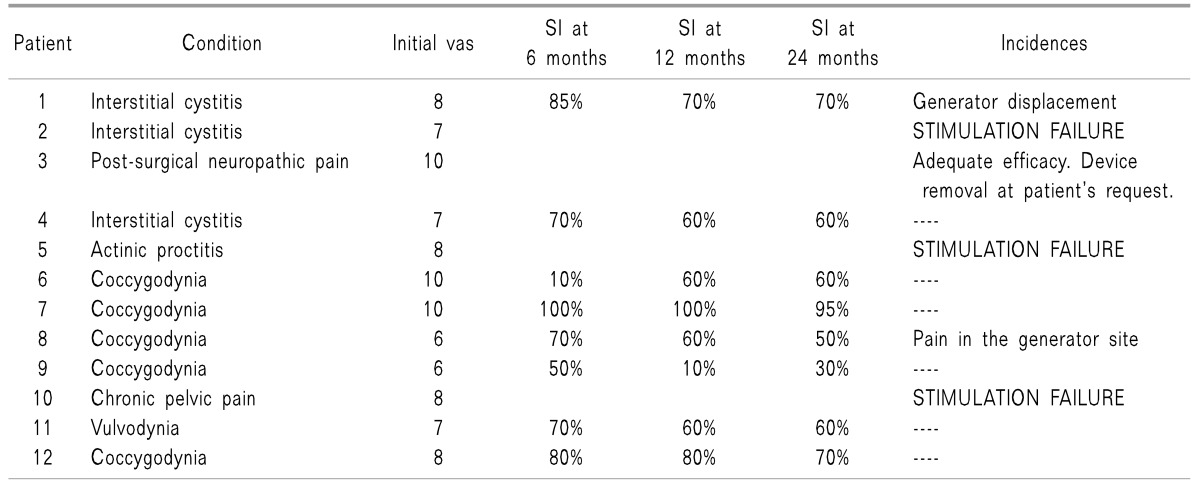
Our experience included 12 patients suffering from conditions with different etiologies that cause chronic pain at the perineal level. Results are presented in Table 1.
Our group of patients included 10 women and two men, with a mean age of 60 years. Their chief complaint was perineal pain, and the average pain duration was 21 months.
After failure with traditional treatment, spinal cord stimulation therapy at the sacral level was determined to be the indicated therapeutic option, and patients were selected for InterStim implantation with a caudal approach.
We evaluated the effectiveness of the therapy based on reduction of pain on the Visual Analogue Scale (VAS) and overall patient satisfaction given their perception of SI.
In eight of the 12 included patients, the initial therapy was effective, and implantation of the final system was performed.
One of the four remaining patients decided to undergo removal of the device, even though there was good efficacy. In the other three patients, the stimulation achieved was not satisfactory, so we decided to remove the electrode after the testing phase.
During subsequent patient follow-up, we obtained similar results regarding efficacy to those usually achieved with other types of electrodes in our unit.
The subjective perception of professionals in our unit regarding InterStim implantation through the caudal pathway is that it offers simplicity and safety.
To date, no cases of electrode displacement have emerged.
Regarding device removal, in those cases in which it has been necessary, the procedure has been conducted without any complications; however, the electrode fixation system requires a careful removal, with rotational movements.
In terms of complications caused by the procedure, two of the patients reported discomfort or pain in the area where the generator had been placed during the first year after implantation; in one case, a slight displacement of the generator could be observed, so it was necessary to revise the site and replace the generator.
Neuromodulation at the sacral level has been widely documented as a beneficial form of therapy for patients with urological conditions, primarily those with chronic urinary bladder dysfunction refractory to traditional treatments. Results in patients with proctological conditions have also been satisfactory [12389101112].
Sacral root stimulation is a well-tolerated therapy, with few complications; moreover, it is a relatively easy technical procedure in expert hands, and therefore has become a widely used treatment among the population.
Neuromodulation treatment is usually offered to patients who suffer from conditions causing chronic pain, after these patients have experienced a lack of success with multiple traditional treatment options.
In our experience, at the time of accepting this therapy, many patients are experiencing complicated psychological, socio-occupational, and family situations, as a direct consequence of their conditions; therefore, in addition to seeking an effective solution to their medical problems, with an acceptable reduction in pain, we must try to ensure that the therapy offered is as final as possible, thus minimizing the number of additional procedures required.
For this reason, when we began using the caudal approach to inserting anterograde electrodes for sacral stimulation, regardless of the technical simplicity of the procedure and the good results achieved in stimulation, our satisfaction was partial, because in our patients as well as in the case series published in the literature (rate of catheter movement, 2%-18%), there was an unacceptable rate of catheter movement, and therefore treatment failure [12].
Each migration of a stimulation electrode which had previously been effective represents a step back in the patient's recovery, because in most cases, given the good results of the therapy, patients have already become "dependent" on it, to a certain extent. On the other hand, the prospect of considering a new procedure to place the electrode again entails an increase in economic costs, which we must try to minimize as much as possible.
Our interest in using the caudal approach for sacral root stimulation is based primarily on our experience and the low rate of complications. Even though we employ other methods of approach with relative frequency, such as the transsacral pathway, we consider caudal punctures easier and safer, and believe that we will achieve a faster puncture procedure and more adequate electrode placement compared to the use of other approaches.
Given that local anesthesia is used in most cases for patients undergoing the insertion of spinal stimulation electrodes, and that there is frequently no sedative treatment (because patient collaboration is essential to determine the effectiveness of stimulation), we consider it important to choose the approach and technique which will allow us to be as effective and rapid as possible, in order to minimize the time required for surgery.
Starting to use an InterStim electrode with a caudal approach is an option which will allow us to attempt to achieve all of our objectives in the therapy: An effective neuromodulation for our patient's condition, ease of insertion, and a reduction in the rate of electrode migration, given that the InterStim features its own anti-movement system.
One of our doubts when we began implanting the sacral electrode through this new approach, for which it was not initially designed, was whether it would cause any type of problems when faced with the hypothetical need to remove it. Our experience has demonstrated that InterStim removal is easy, and does not lead to any problems; however, due to its fixation mechanism, it is true that removal must be conducted by exerting traction at the same time as rotational movements of the electrode.
Sacral root nerve stimulation has positioned itself as an effective therapy for chronic pelvic and perineal pain.
The options for a pelvic root approach are varied, and in our experience, the caudal approach is one of the safest and most simple and effective options. Among the problems presented by this approach, the most frequent is the displacement of the electrode from its initial situation, with the subsequent loss of therapeutic effectiveness.
The caudal insertion of the InterStim electrode, with its own fixation system, and initially designed for transsacral insertion, appears in our experience to be a satisfactory option which can minimize electrode displacement, achieving similar results in terms of therapeutic efficacy, and causing no difficulties in terms of its removal.
References
1. Aló KM, McKay E. Selective Nerve Root Stimulation (SNRS) for the treatment of intractable pelvic pain and motor dysfunction: a case report. Neuromodulation. 2001; 4:19–23. PMID: 22151567.

2. Alo KM, Yland MJ, Redko V, Feler C, Naumann C. Lumbar and sacral nerve root stimulation (NRS) in the treatment of chronic pain: a novel anatomic approach and neuro stimulation technique. Neuromodulation. 1999; 2:23–31. PMID: 22151059.

3. Feler CA, Whitworth LA, Fernandez J. Sacral neuromodulation for chronic pain conditions. Anesthesiol Clin North America. 2003; 21:785–795. PMID: 14719719.

4. Kohli N, Rosenblatt PL. Neuromodulation techniques for the treatment of the overactive bladder. Clin Obstet Gynecol. 2002; 45:218–232. PMID: 11862074.

5. Chodez M, Trilling B, Thuillier C, Boillot B, Barbois S, Faucheron JL. Results of sacral nerve neuromodulation for double incontinence in adults. Tech Coloproctol. 2014; 18:1147–1151. PMID: 25380739.

6. Steinberg AC, Oyama IA, Whitmore KE. Bilateral S3 stimulator in patients with interstitial cystitis. Urology. 2007; 69:441–443. PMID: 17382139.

7. El-Gazzaz G, Zutshi M, Salcedo L, Hammel J, Rackley R, Hull T. Sacral neuromodulation for the treatment of fecal incontinence and urinary incontinence in female patients: long-term follow-up. Int J Colorectal Dis. 2009; 24:1377–1381. PMID: 19488765.

8. Buffenoir K, Rioult B, Hamel O, Labat JJ, Riant T, Robert R. Spinal cord stimulation of the conus medullaris for refractory pudendal neuralgia: a prospective study of 27 consecutive cases. Neurourol Urodyn. 2015; 34:177–182. PMID: 24249588.

9. Hunter C, Davé N, Diwan S, Deer T. Neuromodulation of pelvic visceral pain: review of the literature and case series of potential novel targets for treatment. Pain Pract. 2013; 13:3–17. PMID: 22521096.

10. Kim JH, Hong JC, Kim MS, Kim SH. Sacral nerve stimulation for treatment of intractable pain associated with cauda equina syndrome. J Korean Neurosurg Soc. 2010; 47:473–476. PMID: 20617098.

11. Fariello JY, Whitmore K. Sacral neuromodulation stimulation for IC/PBS, chronic pelvic pain, and sexual dysfunction. Int Urogynecol J. 2010; 21:1553–1558. PMID: 20972541.

12. Mayer RD, Howard FM. Sacral nerve stimulation: neuromodulation for voiding dysfunction and pain. Neurotherapeutics. 2008; 5:107–113. PMID: 18164489.

13. Feler CA, Whitworth LA, Brookoff D, Powell R. Recent advances: sacral nerve root stimulation using a retrograde method of lead insertion for the treatment of pelvic pain due to interstitial cystitis. Neuromodulation. 1999; 2:211–216. PMID: 22151210.

14. Aló KM, Zidan AM. Selective nerve root stimulation (SNRS) in the treatment of end-stage, diabetic, peripheral neuropathy: a case report. Neuromodulation. 2000; 3:201–208. PMID: 22151525.

15. Aló KM, Gohel R, Corey CL. Sacral nerve root stimulation for the treatment of urge incontinence and detrusor dysfunction utilizing a cephalocaudal intraspinal method of lead insertion: a case report. Neuromodulation. 2001; 4:53–58. PMID: 22151611.

16. Richter EO, Abramova MV, Aló KM. Percutaneous cephalocaudal implantation of epidural stimulation electrodes over sacral nerve roots--a technical note on the importance of the lateral approach. Neuromodulation. 2011; 14:62–67. PMID: 21992164.

17. Thon WF, Baskin LS, Jonas U, Tanagho EA, Schmidt RA. Surgical principles of sacral foramen electrode implantation. World J Urol. 1991; 9:133–137.

18. Siegel SW, Catanzaro F, Dijkema HE, Elhilali MM, Fowler CJ, Gajewski JB, et al. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology. 2000; 56(Suppl 1):87–91. PMID: 11114569.

19. Brazzelli M, Murray A, Fraser C. Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. J Urol. 2006; 175:835–841. PMID: 16469561.

20. van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á, Siegel S, Jonas U, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007; 178:2029–2034. PMID: 17869298.

21. Park CH, Kim BI. Sacral nerve stimulation through the sacral hiatus. Korean J Pain. 2012; 25:195–197. PMID: 22787552.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download