Abstract
Background
Chemotherapy-induced peripheral neuropathy is a major side effect of anti-cancer drugs, and our knowledge of its mechanisms is lacking. Several models for chemotherapy-induced neuropathy have been introduced. However, the outcomes of these models differ significantly among laboratories. Our object was to create a model of chemotherapy-induced neuropathy in rats with cancer.
Methods
Female Sprague-Dawley rats were used. Mammary rat metastasis tumor (MRMT-1) cells were implanted subcutaneously in rats. Chemotherapy-induced peripheral neuropathy was induced by injection of cisplatin once a day for four days. The responses to mechanical and thermal stimuli were examined using von Frey filaments, acetone, and radiant heat.
Results
Cisplatin (2 mg/kg/day) produced mechanical allodynia, while it did not induce cold allodynia or thermal hyperalgesia. This dose of cisplatin could work successfully against cancer. Body weight loss was not observed in cisplatin-treated rats, nor were other abnormal behaviors noted in the same rats.
The majority of patients with cancer are treated with chemotherapy [1]. However, side effects of chemotherapy often limit its use. Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect experienced by patients following exposure to chemotherapeutic agents. The incidence of CIPN can be as high as 80 to 90% of patients receiving chemotherapy [2]. CIPN is usually very debilitating, and the pain may be unbearable [3], which may lead to patients being unable to complete the full or optimal treatment schedule. These unwanted events result in a major impact on patients' quality of life, leading to higher levels of negative states such as depression, anxiety, and fear [3]. For these reasons, many researchers have tried to overcome CIPN.
To date, various types of animal models of CIPN have been developed [456]. Although the models all represent CIPN, the characteristics of each differ from the others. Such discrepancies may be caused by the use of different drugs, the routes of drugs administered, the kinds of animals used, and the types of measurements made. Furthermore, we had a specific focus; most investigators have used normal animals without cancer. Therefore, they could not evaluate the anticancer effect of experimental doses of the chemotherapeutic agents on tumors. Considering the serious aspect of the reproducibility of animal modeling, it is important to determine the dosage of chemotherapeutic agents required to induce a model of CIPN. It should be sufficient to produce not only the anticancer effect but also neuropathic pain.
The purpose of this study was to establish a more proper and convenient model of CIPN. First, we observed the development of CIPN with an anticancer drug, cisplatin, in normal rats. Second, we examined the anticancer effect of cisplatin in a bone tumor model by implantation of mammary rat metastasis tumor (MRMT-1) cells, and observed the features of CIPN in the same condition. Finally, we aimed to validate the new CIPN rat model induced by cisplatin through these experiments.
Sprague-Dawley (SD) male rats weighing 100-120 g and female rats weighing 140-160 g were used in the experiments. The female rats were used for the MRMT-1 implantation model. The body weight of the female rats was determined by a previous pilot study to optimize tumor cell growth. The animals were acclimated to the laboratory environment for 5-7 days before use. While in their home cage environment, they were allowed free access to a standard rat diet and tap water. The room was maintained at 20-23℃ with a 12 h/12 h light/dark cycle. The study proposal was reviewed and approved by the Institutional Animal Care and Use Committee.
Syngeneic MRMT-1 rat mammary gland carcinoma cells were cultured in 500 ml of RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum, L-glutamine (final concentration 2 mM), and antibiotic solution (100 U/ml penicillin and 100 g/ml streptomycin sulfate) (all from Gibco BRL Co., Ltd., Grand Island, NY, USA). The cells were released from the tissue culture flasks using 0.1% trypsin (Gibco) and prepared for injection by centrifuging them for 4 min at approximately 1200 rpm. The resulting pellet was resuspended in medium and the cells were counted using a hemocytometer. The cells were then diluted to achieve the desired final concentrations for injection and kept on ice until needed.
Cisplatin (Tocris Cookson Ltd., Bristol, Avon, UK) was dissolved in 0.4% dimethyl sulfoxide (DMSO) in saline just before administration, to a final concentration of 0.2 mg/ml, 0.1 mg/ml or 0.05 mg/ml. The volume for intraperitoneal injection was 1 g body weight per 0.01 ml. The control group received 0.4% DMSO in saline.
The experiments were composed of two sets. The purpose of the first series of the study was to observe the effect of intraperitoneal cisplatin (0.5, 1, or 2 mg/kg) or saline in naive male rats. The purpose of the second was to observe the effect of intraperitoneal cisplatin (2 mg/kg) or saline in female rats with cancer. For tumor cell implantation, 1 × 105 tumor cells were subcutaneously injected into the right back of the rat. For the control group for the tumor cell rats, culture medium was injected in the same fashion. Two weeks after tumor cell or medium implantation, cisplatin or saline was administered. Cisplatin was administered once a day for four days. To 21 days from the start of cisplatin administration, tests for mechanical and thermal allodynia and thermal hyperalgesia were performed and general behaviors were observed. Tumor size was measured 14 and 28 days (14 days after cisplatin administration) after tumor cell implantation. All values in the nociceptive behavioral tests were the mean figure of the responses from each of the hindpaws.
The paw withdrawal threshold (PWT) in response to mechanical stimulation was measured using the up and down method [7] by applying calibrated von Frey filaments from underneath the cage through openings in the mesh floor to the hindpaw. Rats were placed in a separate transparent Plexiglass chamber with a wire mesh floor underneath and acclimated to the test chamber for 30 min. A series of eight von Frey filaments (0.4, 0.7, 1.2, 2.0, 3.6, 5.5, 8.5, and 15 g) were applied vertically to the plantar surface of the hindpaw for 5 s while the hair was bent. Brisk withdrawal or paw flinching was considered a positive response. If a rat showed a positive response, the less stiff filament was used for the next trial, while the stiffer one was used in cases when rats showed no withdrawal or licking. The cutoff value was determined as 15 g, where the PWT was 15 g if a rat did not show any withdrawal or licking response to the application of a 15 g von Frey filament.
Thermal allodynia was measured using the acetone spray test. With the animal atop the wire mesh floor, a drop (0.05 ml) of acetone was placed against the center of the ventral side of the hindpaw and a stopwatch was started. In the following 20 s after acetone application, the rat's response was monitored. Responses to acetone were graded according to the following 4-point scale: 0, no response; 1, quick withdrawal, flick or stamp of the paw; 2, prolonged withdrawal or repeated flicking (≥ 2) of the paw; 3, repeated flicking of the paw with licking directed at the ventral side of the paw. Acetone was applied alternately three times to each paw and the responses were scored categorically. Cumulative scores were then generated by adding the six scores for each rat together, the minimum score being 0 (no response to any of the six trials) and the maximum possible score being 18 (repeated flicking and licking of paws on each of the six trials) [8].
Paw withdrawal latency to radiant heat was assessed as previously described [7]. Each animal was loosely restrained beneath a plastic box on a glass surface and allowed to acclimate for at least 15 min once per day for one week prior to behavioral testing. Testing comprised alternate presentations of radiant heat from below to each hindpaw, focused on the mid-plantar surface. The heat source cut off automatically with paw withdrawal and the latency was recorded. Paw withdrawal latency was defined as the mean of three trials applied to each paw. A cutoff stimulation time of 20 s was used in the event of no movement to avoid skin damage.
Body weight was monitored for 21 days after injection of cisplatin in both normal and cancer rats. Other abnormal behaviors such as hair loss and sedation were also assessed.
Data is expressed as mean ± standard error of the mean (SEM). Time response data are presented as PWT to mechanical stimulation, while dose-response data are presented as the percent of maximum possible effect (%MPE). Withdrawal threshold data from von Frey filament testing were converted to %MPE, according to the formula: %MPE = [(post drug threshold - post-injured baseline threshold) / (cutoff threshold - post-injured baseline threshold)] × 100. To analyze the 50% probability PWT data and dose-responsiveness, one-way analysis of variance (ANOVA) with Scheffe's multiple comparison test was used. P values of < 0.05 were considered statistically significant.
The administration of cisplatin decreased body weight only at the highest dose (2 mg/kg) in normal rats, but body weight returned to baseline after 3 weeks (Fig. 1). This reduction was not seen in rats with tumors implanted. No rats revealed overt abnormal behaviors after cisplatin injection.
In normal rats, 0.5 mg/kg (n = 6) of cisplatin did not alter the withdrawal thresholds compared to baseline. A dose of 1 mg/kg (n = 5) of cisplatin produced a decreasing trend, but this finding was not significant. All rats (n = 6) that received 2 mg/kg of cisplatin showed a powerful reduction of the withdrawal thresholds. In the second series of the study, both rats that received media (n = 5) and those that received tumor cells (n = 5) showed a significant decrease of the withdrawal thresholds in cases of cisplatin injection. However, there was no significant difference in the thresholds between the media and tumor cell groups after cisplatin injection. Saline injection failed to affect the withdrawal thresholds in all rats (Fig. 2).
Tumors developed and their size increased after the implantation of MRMT-1 cells (14 days). After the administration of cisplatin (2 mg/kg), the tumor size decreased gradually over a period of 20 days. In the vehicle group, tumor size increased consistently during the same period (Fig. 5).
CIPN is the chief dose-limiting side effect associated with the major classes of frontline drugs, including the taxanes, the vinca alkaloids, and the platin-based drugs, that are used against all of the most common types of cancer [9]. As a consequence of recent developments in palliative therapy for malignant cancer, a number of patients who are scheduled to undergo chemotherapy will be exposed to this problem. Moreover, the symptoms of CIPN, such as numbness, tingling, burning pain, and sensory-motor impairments, are largely refractory to treatment and often persist as a chronic condition long after treatment. Consequently, CIPN will be a major issue affecting the quality of life and return to productivity in cancer patients [10].
Cisplatin (cis-diamminedichloroplatinum II) is the first member of a class of platinum-containing anti-cancer drugs which have their effect by causing crosslinking of DNA, leading to apoptosis [6]. It is used to treat various types of cancers, including sarcomas, some carcinomas, lymphomas, and germ cell tumors [11]. Unfortunately, the platinum derivative drugs have a molecular affinity for the peripheral nervous system that lacks a vascular barrier, leading to severe peripheral neurotoxicity that affects most cancer patients treated with cisplatin-based chemotherapy [12].
There are many studies that have shown that cisplatin induces peripheral neuropathy in rats or mice [456]. However, these studies were performed with animals in a non-cancerous state. Although cisplatin successfully produced neuropathy using its own study protocol in the CIPN models of primor studies, it is ambiguous whether such regimens of cisplatin may work effectively against cancer. Therefore, we focused on the point that a rat with tumor cells implanted would be a more reasonable model to determine of the adequate cisplatin dose as well as a more appropriate model for reproduction of the clinical situation. Conditions are significantly different from normal to cancer, especially in the immune system. For example, Dunn et al. reported that a number of advanced cancer patients show an immunosuppressive state with decreased T-cell activity [13]. So, we had assumed that the symptoms of CIPN would be different between normal rats and those with cancer.
In this study, we found that administration of intraperitoneal cisplatin once a day for four days decreased the paw mechanical withdrawal threshold in both normal rats and those with induced tumors. In the normal rat model, mechanical allodynia developed in a dose-dependent manner. These findings demonstrate that the peripheral neuropathy produced by cisplatin is dose-limited. In the tumor model, a dose of 2 mg/kg of intraperitoneal cisplatin produced neuropathic pain and decreased the tumor size effectively. However, regarding tactile allodynia, there was no significant difference between the tumor cell and media groups.
This study has some limitations. First, we did not check immunologic parameters in the group of rats with tumors. Consequently, we could not assess the comparative immunologic status of each group of rats. In addition, we used syngeneic MRMT-1 rat mammary gland carcinoma cells to make the cancer rat model. It is probable that there might be a different process in the systemic immune response against natural born cancer cells and implanted tumor cells. Duration is also likely a factor. In this study, tumor cells were installed for 14 days after implantation, and this might not be long enough to change the immunologic response of rats. Second, the rat model after administration of 2 mg/kg cisplatin in this study did not show thermal hyperalgesia or allodynia. In contrast, in a previous study of a CIPN model with a mouse, cisplatin produced tactile allodynia and thermal hyperalgesia when used at total doses up to 15 mg/kg with a delivery period of over 20 days [1415]. As compared with the prior study, we presumed that our dose and duration of treatment may be too low and short to induce thermal hyperalgesia and allodynia. Third, we used female rats for the tumor cell implantation. With so many variables mainly due to sexual hormones, it is generally accepted that female rats are not suitable for pain modeling. Thus, it is appropriate to compare behavioral tests between male rat tumor models and naïve models. Lastly, we used only behavioral tests as an outcome measure method. Clinically, the majority of patients suffer from spontaneous painful paresthesia. However, it is difficult to evaluate paresthesia with behavioral tests that measure evoked responses.
We could not validate a significant difference in mechanical allodynia between the group with implanted cancer and the control group in this study. However, in spite of the limitations, this study is worthwhile in its identification of an optimal cisplatin dose (can be used as a lowest limit) in a rat model of CIPN. For further improvement of this model, more studies regarding cancer immunology and chemotherapy are needed. In addition, standardization of protocols (genetic background of animal, dose and period of treatment, outcome measure methods for evaluation of neuropathy) is necessary for appropriate CIPN modeling. We hope that our work will lead to the new beginning of the CIPN rat model and that the type of model used in the current study will be widely used in future studies to elucidate the mechanism of cisplatin-induced peripheral neuropathy and lead to the discovery of new drugs for the treatment of neuropathic pain.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI12016-1) Chonnam National University Hospital Biomedical Research Institute.
References
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008; 13:27–46. PMID: 18346229.

2. Höke A. Animal models of peripheral neuropathies. Neurotherapeutics. 2012; 9:262–269. PMID: 22415319.

3. Franconi G, Manni L, Schröder S, Marchetti P, Robinson N. A systematic review of experimental and clinical acupuncture in chemotherapy-induced peripheral neuropathy. Evid Based Complement Alternat Med. 2013; 2013:516916. PMID: 23983788.

4. Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009; 6:620–629. PMID: 19789067.

5. Cavaletti G, Petruccioli MG, Tredici G, Marmiroli P, Barajon I, Fabbrica D, et al. Effects of repeated administration of low doses of cisplatin on the rat nervous system. Int J Tissue React. 1991; 13:151–157. PMID: 1960015.
6. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008; 44:1507–1515. PMID: 18571399.

7. Jeong S, Lee SH, Kim YO, Yoon MH. Antinociceptive effects of amiloride and benzamil in neuropathic pain model rats. J Korean Med Sci. 2013; 28:1238–1243. PMID: 23960454.

8. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994; 59:369–376. PMID: 7708411.

9. Marmiroli P, Nicolini G, Miloso M, Scuteri A, Cavaletti G. The fundamental role of morphology in experimental neurotoxicology: the example of chemotherapy-induced peripheral neurotoxicity. Ital J Anat Embryol. 2012; 117:75–97. PMID: 23420996.
10. Rostock M, Jaroslawski K, Guethlin C, Ludtke R, Schröder S, Bartsch HH. Chemotherapy-induced peripheral neuropathy in cancer patients: a four-arm randomized trial on the effectiveness of electroacupuncture. Evid Based Complement Alternat Med. 2013; 2013:349653. PMID: 24066010.

11. Al Moundhri MS, Al-Salam S, Al Mahrouqee A, Beegam S, Ali BH. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: some behavioral, biochemical, and histopathological studies. J Med Toxicol. 2013; 9:25–33. PMID: 22648527.

12. Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg. 2013; 116:224–231. PMID: 23223118.

13. Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006; 6:836–848. PMID: 17063185.

14. Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003; 182:12–20. PMID: 12821373.

15. Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain. 2009; 5:9. PMID: 19245717.

Fig. 1
Body weight changes were measured after administration of different doses of cisplatin or vehicle (A), MRMT-1 or media injection before cisplatin or vehicle groups (B). Each line represents mean ± SEM. B: baseline body weight before administration of cisplatin or vehicle. *P < 0.05.
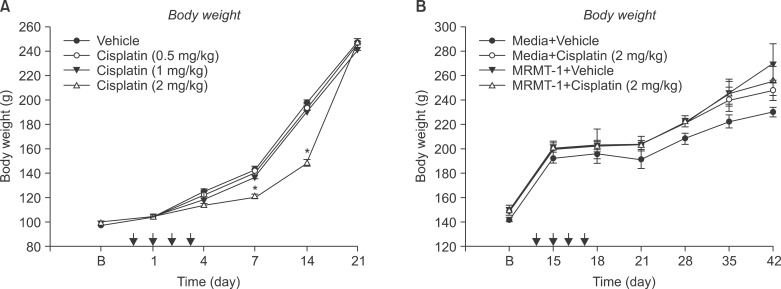
Fig. 2
Hindpaw withdrawal responses to von Frey filaments were measured after administration of different doses of cisplatin or vehicle (A), MRMT-1 or media injection before cisplatin or vehicle groups (B). Each line represents mean ± SEM. B: baseline withdrawal threshold before administration of cisplatin or vehicle. *P < 0.001.
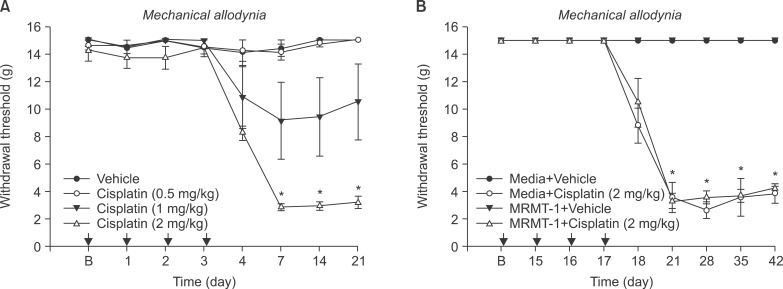
Fig. 3
Hindpaw withdrawal responses to acetone were measured after administration of different doses of cisplatin or vehicle (A), MRMT-1 or media injection before cisplatin or vehicle groups (B). Each line represents mean ± SEM. B: baseline thermal allodynia before administration of cisplatin.
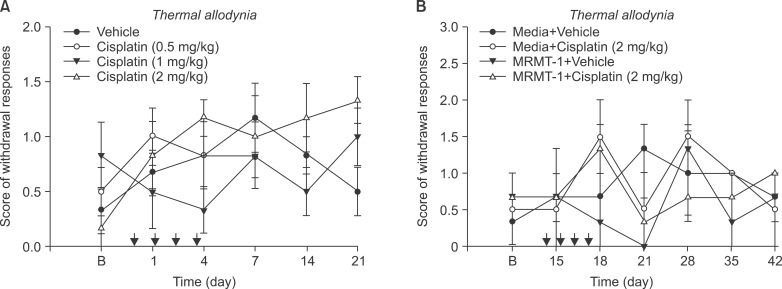
Fig. 4
Hindpaw withdrawal responses to radiant heat were measured after administration of different doses of cisplatin or vehicle (A), MRMT-1 or media injection before cisplatin or vehicle groups (B). Each line represents mean ± SEM. B: baseline thermal hyperalgesia before administration of cisplatin or vehicle.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download