Abstract
Background
Good postoperative pain control is an important part of adequate postoperative care. Patient-controlled epidural analgesia (PCEA) provided better postoperative analgesia compared to other conventional analgesic methods, but several risks have been observed as well. We therefore surveyed the efficacy and safety of PCEA in this retrospective observational study.
Methods
We analyzed collected data on 2,276 elective surgical patients who received PCEA with ropivacaine and fentanyl. Patients were assessed by a PCA service team in the post-anesthesia care unit (PACU), at 1-6 h, 6-24 h, and 24-48 h postoperatively for adequate pain control. The presence of PCEA-related adverse events was also assessed.
Results
Numerical pain score (median [interquartile range]) were 3 [1-4], 5 [4-7], 4 [3-5], and 3 [3-5] in the PACU, at 1-6 h, 6-24 h, and 24-48 h postoperatively. Median pain scores in patients underwent major abdominal or thoracic surgery were higher than other surgical procedure in the PACU, at 1-6 h after surgery. Nausea and vomiting (20%) and numbness and motor weakness (15%) were revealed as major PCEA-related adverse events during the postoperative 48 h period. There were 329 patients (14%) for whom PCEA was ceased within 48 h following surgery.
Conclusions
Our data suggest that the use of PCEA provides proper analgesia in the postoperative 48 h period after a wide variety of surgical procedures and that is associated with few serious complications. However, more careful pain management and sustainable PCEA monitoring considering the type of surgical procedure undergone is needed in patients with PCEA.
The provision of high-quality analgesia in the postoperative period is important, not only to relieve post-surgical pain and improve well-being, but also because inadequate pain control may increase morbidity, lead to prolonged hospital stays, and increase medical costs [1,2]. Patient-controlled epidural analgesia (PCEA) is a widely used postoperative analgesic strategy because it is an effective and safe method of acute postoperative pain relief [3-5]. Previous large-scale analysis has demonstrated that PCEA provides better postoperative analgesia compared to parenteral opioids or intravenous patient-controlled analgesia (IV-PCA) [3,6]. However, severe complications of PCEA, such as respiratory depression, epidural hematoma, and catheter-related infections can threaten the safety of patients [6]. Moreover, early cessation of PCEA due to side effects or complications may not only lead to patient discomfort, but also to dissatisfaction with postoperative pain management and an increase in medical costs as well [7]. Therefore, an investigation of the actual conditions of PCEA use is clinically significant. There are now several published studies detailing experiences with large numbers of patients who received postoperative PCEA [8-10]. Unfortunately, there have not been any published studies detailing the efficacy and safety of PCEA using a large database in Korea. We thus evaluated the efficacy and safety of PCEA using our large institutional database. This study is a retrospective observational audit of 2276 patients who received PCEA for postoperative analgesia in general surgical wards in Korea.
This study was approved by our hospital's Institutional Review Board (ref. 4-2012-0201). The sample population was defined as elective surgical patients who received PCEA with ropivacaine and fentanyl for postoperative pain control between September 2010 and December 2011 at our hospital. Exclusion criteria were the following: age less than 18 years, other regimen of PCEA, and requirement of postoperative ventilator support or intensive care.
Epidural catheters were inserted before or after surgery at a vertebral level corresponding to the dermatomal level of the surgical incision. An 18-G Tuohy needle and 20-G epidural catheter were used (B. Brown Co., PA, USA). A loss of resistance technique was used to identify the epidural space, and the epidural catheter was inserted to the proper depth in the epidural space. Adhesive tape (Tegaderm; 3M, MN, USA) was used to affix the catheter along the patient's back, followed by an aseptic dressing. General or regional (spinal) anesthesia was conducted at the discretion of attending anesthesiologists. All patients used a disposable PCA pump (Accufuser plus®; Woo Young Medical, Korea), which was programmed to deliver 5 ml/hr (or 2 ml/hr) as a background infusion and 2.0 ml (or 0.5 ml) on demand, with a 15 min lockout during a 48 h period. The PCA regimen typically composed of fentanyl (2-5 µg/ml) plus ropivacaine (0.15-0.20%). The PCA service team, which was comprised of anesthesiologists and PCA nurse specialists, monitored patients in the post-anesthesia care unit (PACU), at 1-6, 6-24, and 24-48 h intervals after surgery to inquire about the occurrence of adverse events, and the needs for rescue IV analgesics (ketolorac, tramadol, meperidine). PCEA-related adverse events included numbness, motor weakness, nausea and vomiting, sedation, dizziness, headache, hypotension (systolic blood pressure < 90 mmHg), pruritus, infection, respiratory depression and mechanical catheter problems. In addition to these parameters, patients were asked to rate their worst pain intensity during each time period. Pain intensity scores were measured on a numerical rating scale (0 = no pain to 10 = worst pain imaginable). The PCEA was stopped for approximately 2 h if side effects or complications of PCEA were not relieved by any treatments, and the PCEA was completely discontinued when side effects or complications persisted with the resumption of PCEA and/or at the request of the patient. The PCA service team recorded the reason for cessation, time of cessation, adverse side effects, and residual volume of PCEA if it was discontinued within 48 h of surgery. Elective completion of PCEA is defined as PCEA being maintained during postoperative 48 h until the PCEA was finished. Additional patient data that were collected included age, gender, height, weight, body mass index (BMI), medical history, duration of anesthesia, type of anesthesia, and type of surgery.
Corrections and insertion of missing data were performed by checking the original documentation sheets or hospital medical records. Continuous variables are shown as mean ± SD, and categorical variables are shown as a number (percentage). Nonparametric data were analyzed using Fisher's exact test or the chi-square test. Because pain intensity may depend on the type of surgery, pain scores were compared in subgroup analyses. Owing to the fact that the data were not normally distributed, the Kruskal-Wallis test and the Friedman test with post-hoc testing using the Wilcoxon rank-sum test were used to compare groups. Statistical analysis was performed with SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA). Values of P < 0.05 were considered statistically significant.
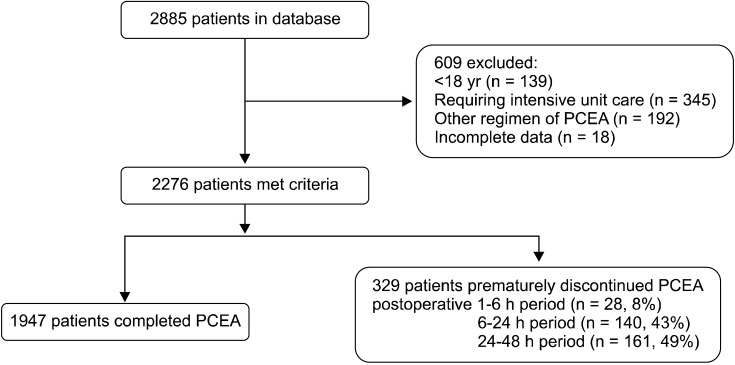
A total of 2,885 patients were assessed for study eligibility, of whom 2,276 met study criteria and were finally analyzed in this observational study. There were 329 patients (14%) for whom PCEA was terminated within 48 h after surgery due to PCEA-related adverse events. In the cessation population, PCEA was ceased in 8%, 43%, and 49% of patients at 1-6, 6-24, and 24-48 h after surgery, respectively (Fig. 1).
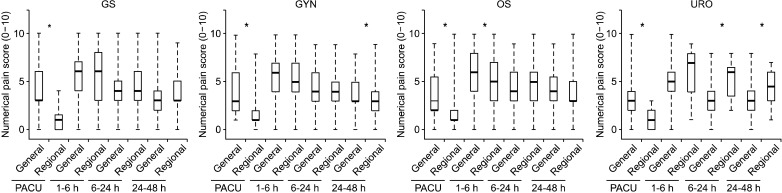
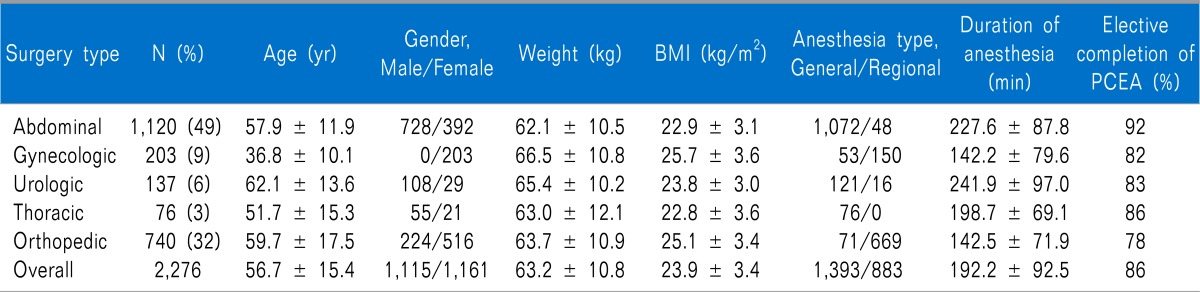
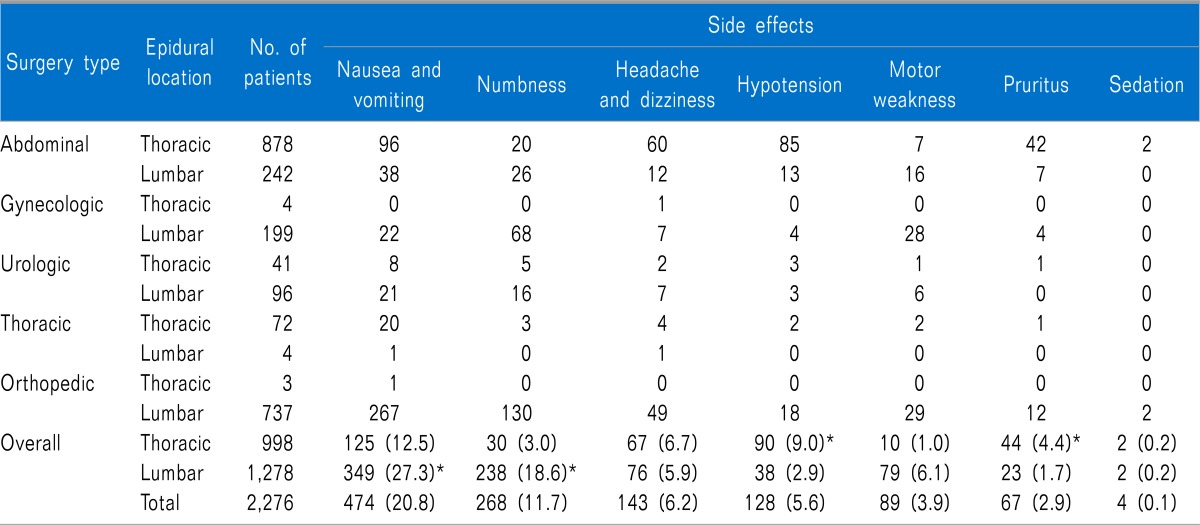
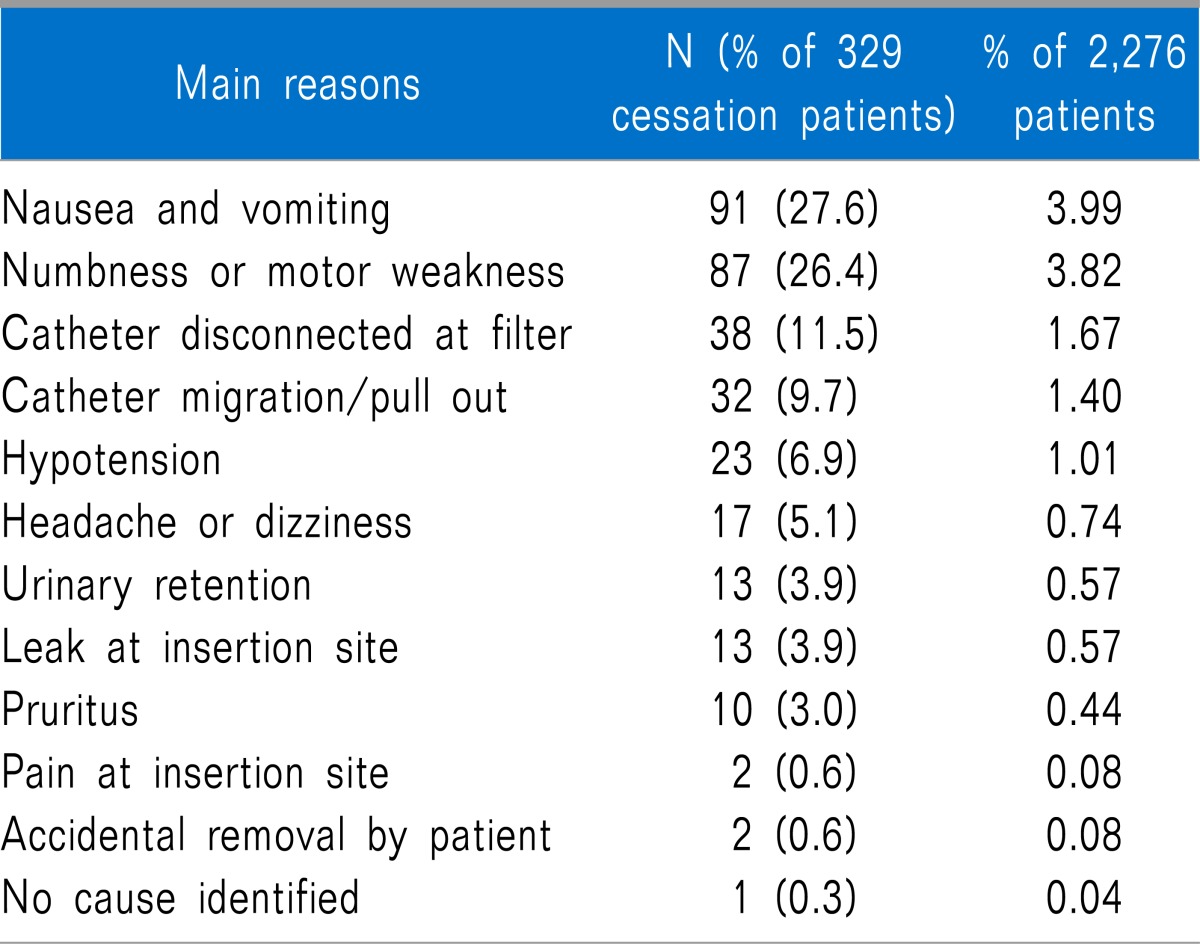
The type of surgery the patients received is listed in Table 1, along with patients characteristics, anesthesia type, duration of anesthesia, and elective completion rate of PCEA for each surgery type. Completion of PCEA (continuing until the PCEA was finished) was elective in 86% of patients in the postoperative 48 h. Patients who underwent orthopedic surgery showed the lowest elective completion rate of PCEA. The mean concentration of fentanyl and ropivacaine were 3.04 ± 1.87 µg/ml and 0.16 ± 0.04%, respectively. A PCA device with a 5 ml/hr infusion rate was used in 91% of patients and a device with a 2 ml/hr rate was used in 9%. There were no statistically significant differences in pain scores (3 [1-4] vs. 3 [1-4], 5 [4-7] vs. 5 [4-6], 4 [3-5] vs. 4 [3-5], and 3 [3-5] vs. 3 [3-5]) and cessation rate (12% vs. 14%, P = 0.091) between the two types of PCA device (infusion rate 2 ml/hr vs. 5 ml/hr). The fentanyl dose delivered hourly was 13.3 ± 5.6 µg/hr. Numerical pain scores with the median and the inter-quartile range according to type of surgical procedure in the PACU, at postoperative 1-6, 6-24, and 24-48 h assessment intervals are shown in Fig. 2. In the PACU and the postoperative 1-6 h period, median pain scores in patients who underwent major abdominal surgery or thoracic surgery were higher than those for other surgical types (P < 0.05 using the Friedman test with post-hoc testing using the Wilcoxon rank-sum test with the Bonferroni correction). The percentages of patients who received rescue analgesic were 15%, 55%, 41%, and 15% at each assessment intervals. The percentages of patients with clinically adequate analgesia (numerical pain score ≤ 3) were 71%, 38.6%, 61.1%, and 72.8% at each assessment intervals. Comparison between numerical pain scores according to type of anesthesia in the PACU, at postoperative 1-6, 6-24, and 24-48 h assessment intervals for each surgery type is shown in Fig. 3. Patients who underwent surgery under regional anesthesia showed lower median pain scores than those who underwent general anesthesia in the PACU, postoperative 1-6 h period (P < 0.001). Patients who underwent general anesthesia suffered more postoperative pain than those who underwent regional anesthesia regardless of surgery type. PCEA-related side effects according to type of surgical procedure and the insertion site of PCEA are listed in Table 2. Overall, 474 patients (20.8%) experienced nausea and emetic symptoms (vomiting or retching) during the postoperative 48 h, and the number of patients with nausea or emetic symptoms was 51, 330, 304, an 128 patients at each assessment intervals. The incidence of numbness and motor weakness was significantly higher with lumbar catheters than which thoracic catheters (P < 0.001). Hypotension and pruritus showed significantly higher incidence with thoracic catheters than which lumbar catheters (P < 0.001). Reasons for cessation of PCEA within the postoperative 48 h are listed in Table 3. Cessation of PCEA was defined as patients being unable to maintain PCEA during the postoperative 48 h due to side effects or complications. Nausea/emetic symptoms, numbness/motor weakness, and catheter-related mechanical problems were found to be major causes of premature cessation of PCEA within the postoperative 48 h. Mechanical problems that were observed included catheter migration/pull out (9.7%) and disconnection at the filter (11.5%).
The present study is the largest observational single-center analysis of postoperative PCEA published yet in Korea. Our data suggest that the use of PCEA with ropivacaine and fentanyl provides relatively adequate analgesia in the postoperative 48 h period after a wide variety of surgical procedures and is associated with few serious complications. However, more careful pain management and sustainable PCEA monitoring may be required in patients using PCEA in general surgical wards.
Although PCEA may provide better postoperative analgesia compared with on-demand parental opioids, IV-PCA, or peripheral nerve block, many patients using PCEA also experienced some postoperative pain in the immediate postoperative period [6]. Our data show that pain scores and analgesic requirements were high in the first 24 h following surgery, which is consistent with previous studies [6,8-10]. However, our patients using PCEA suffered more postoperative pain, based on pain scores in the postoperative 24 h period, than was observed in controlled prospective studies conducted in similar PCEA environments [8]. Moreover, patients who underwent major abdominal or thoracic surgery experienced more pain compared with those who underwent other surgical procedures in the immediate postoperative period. There are several explanations for these results. In this study, the lower dose of fentanyl used in the PCEA may contribute to the lower efficacy of pain relief compared to previous study outcomes. The hourly delivered fentanyl dose in our study was 30%-70% of that used in previous studies [8,9]. In addition, the use of a fixed-setting PCA device in our study population might affect pain intensity. Most previous epidural analgesia studies were conducted using adjustable-setting PCA devices, which allow more flexibility in the timing and amount of dose delivered [8-10]. We routinely used a bolus dose of local anesthetics via epidural catheter at the discretion of attending anesthesiologists toward the end of the surgical period [11]. This practice provided more adequate analgesia and led to lower analgesic requirements in patients with PCEA in the PACU. In the same manner, more aggressive pain management via the epidural route is especially needed in patients who undergo major abdominal or thoracic surgery for better postoperative analgesia within the immediate postoperative period.
Our data showed that nausea and vomiting were major PCEA-related side effects and that these resulted in premature cessation of PCEA in many cases. Previous studies reported a high incidence of nausea and vomiting in patients who received epidural analgesia with opioids [8,10]. We are aware of one study regarding PCEA-related nausea and vomiting, in which female gender was suggested as a possible risk factor for PCEA related nausea and vomiting [8]. In our population, 91 patients prematurely discontinued PCEA due to intractable nausea and vomiting, and 65% of these were female. Multimodal prophylactic antiemetic strategies may be considered as strategies for the prevention of PCEA-related nausea and vomiting, especially in female patients. Numbness and motor weakness are the side effects most attributable to epidural analgesia [3]. Thoracic placement of the epidural catheter produced both less numbness and less motor weakness than lumbar placement in our study, which is consistent with previous studies [3,8]. According to previous studies, low lumbar (L4-L5) administration of epidural local anesthetics increased the incidence of lower limbs motor block compared with high lumbar administration (L1-L2), and numbness and motor weakness were significantly associated with the concentration of local anesthetics [12-15]. Additionally, the migration of the epidural catheter toward the nerve root is a possible risk factor for PCEA-related numbness and motor weakness [15]. Therefore, careful placement of the epidural catheter and judicious consideration of the concentration of local anesthetics to be used in the PCEA regimen are required in the lumbar approach. Most previous studies analyzing epidural analgesia observed a wide variation in the incidence/severity of hypotension [3,8-10]. In our study, thoracic epidural analgesia resulted in a higher incidence of hypotension than lumbar epidural analgesia. These results may be explained by an anatomical difference between the thoracic and lumbar epidural spaces [16]. The thoracic epidural space contains less fat, and the thoracic dura is less adhere to the surrounding bony canal than in the lumbar epidural space. Moreover, sympathetic block may aggravate hypotension [6]. We also found that mechanical problems including dislodgement of the catheter and disconnection at the filter accounted for 21% of premature cessation of PCEA. Interestingly, patients who underwent orthopedic or obstetrics and gynecologic surgery, who required early ambulation and exercise in the postoperative period, were especially prone to prematurely cease PCEA due to mechanical problems in our study. The incidence of mechanical problems with PCEA was reported to be approximately 13-23% in large-scale studies [9,17,18]. Various methods of reinforcing the dressing have been described to attempt to reduce and prevent these problems [17]. Other mechanical problems due to occlusion, kinking, or PCA device malfunction may cause accidental discontinuation of PCEA. More meticulous dressing and fixation of the epidural catheter with adhesive materials, as well as sustainable management, will reduce PCEA-related mechanical problems. In addition, we cannot overemphasize the importance of improved education of physicians and nursing staff regarding prevention and management of various mechanical problems related to PCEA. In our study, sedation was observed in 4 (0.2%) patients, without respiratory depression. These patients were slightly drowsy and were managed by a temporary cessation of PCEA. There were no cases of serious epidural catheter-related complications, such as respiratory depression, epidural hematoma or abscess, local inflammation, or permanent neurologic damage in our population. Despite their low incidence, most studies reported these serious complications that could threaten the safety of patients [6,9,10]. These problems are known to closely associate with high opioid infusion or bolus dose and long duration of placement of the epidural catheter [17]. The optimal concentration of fentanyl for PCEA infusion is not known, although the majority of studies dealing with epidural fentanyl report that doses of fentanyl of 4-10 µg/ml and 1 µg/ml in combination with bupivacaine were frequently inadequate analgesia and required high infusion rates [9]. Decreased use of local anesthetics and opioids with PCEA with equivalent analgesia would be valuable if drug-related side effects could be reduced [19]. A highly significant increase in inflammatory complications has been reported in case in which an epidural catheter is in place for four or more days [17]. We used a fixed-setting PCA device with an average fentanyl concentration of 3.0 µg/ml for 2 days postoperatively.
This study has some limitations. Being observational in nature, this study used the real-world clinical practice model in which attending physicians decided on the placement of epidural catheters and the prescription of the PCA regimen, as well as the use and cessation of PCEA on the general ward, according to institutional guidelines. Therefore, the decision to administer the PCA regimen and to cease PCEA could have been influenced by the attending physician's judgment and preconceptions concerning PCEA. Also, we cannot precisely measure ropivacaine and fentanyl requirements in individual patients considering the bolus and infusion doses of PCEA. Therefore, further studies will be required to take these limitations into consideration and conduct more controlled observations of PCEA in Korea.
In conclusion, our study demonstrated that PCEA provides relatively effective and safe postoperative analgesia. More careful management of PCEA considering various individual factors, however, may be needed to ensure maximal benefit for all patients.
ACKNOWLEDGEMENTS
We wish to thank PCA nurse practitioner Won-Hee Baek, R.N. and Sun-Young Oh, R.N. for their expert assistance in collecting data. In addition, we thank Research Instructor Dong Wook Kim, Ph.D., Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea, in the analysis of data in our article.
References
1. Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001; 87:62–72. PMID: 11460814.

2. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003; 97:534–540. PMID: 12873949.

3. Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA Jr, Wu CL. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003; 290:2455–2463. PMID: 14612482.
4. Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995; 82:1474–1506. PMID: 7793661.
5. Kim DH. Effective continuous infusion and bolus doses for patient-controlled epidural analgesia using ropivacaine. J Korean Pain Soc. 2002; 15:75–79.
6. Pöpping DM, Zahn PK, Van Aken HK, Dasch B, Boche R, Pogatzki-Zahn EM. Effectiveness and safety of postoperative pain management: a survey of 18 925 consecutive patients between 1998 and 2006 (2nd revision): a database analysis of prospectively raised data. Br J Anaesth. 2008; 101:832–840. PMID: 18945716.

7. Chen PP, Chui PT, Ma M, Gin T. A prospective survey of patients after cessation of patient-controlled analgesia. Anesth Analg. 2001; 92:224–227. PMID: 11133632.

8. Liu SS, Allen HW, Olsson GL. Patient-controlled epidural analgesia with bupivacaine and fentanyl on hospital wards: prospective experience with 1,030 surgical patients. Anesthesiology. 1998; 88:688–695. PMID: 9523813.

9. Scott DA, Beilby DS, McClymont C. Postoperative analgesia using epidural infusions of fentanyl with bupivacaine. A prospective analysis of 1,014 patients. Anesthesiology. 1995; 83:727–737. PMID: 7574052.
10. de Leon-Casasola OA, Parker B, Lema MJ, Harrison P, Massey J. Postoperative epidural bupivacaine-morphine therapy. Experience with 4,227 surgical cancer patients. Anesthesiology. 1994; 81:368–375. PMID: 8053587.

11. Cha YD, Song JH, Song JH, Kim TJ, Lee HS, Lee CS, et al. Is initial loading dose necessary for continuous epidural analgesia after brief surgery? J Korean Pain Soc. 2000; 13:44–48.
12. Brennum J, Arendt-Nielsen L, Secher NH, Jensen TS, Bjerring P. Quantitative sensory examination in human epidural anaesthesia and analgesia: effects of lidocaine. Pain. 1992; 51:27–34. PMID: 1454401.

13. Liu SS, Moore JM, Luo AM, Trautman WJ, Carpenter RL. Comparison of three solutions of ropivacaine/fentanyl for postoperative patient-controlled epidural analgesia. Anesthesiology. 1999; 90:727–733. PMID: 10078673.

14. Moon DE, Shim JY, Lim YG, Kim YS, Kim BC, Kim SN. Neurologic complications following epidural analgesia. J Korean Pain Soc. 1997; 10:291–295.
15. Talu GK, Erdine S. Complications of epidural neuroplasty: a retrospective evaluation. Neuromodulation. 2003; 6:237–247. PMID: 22151070.

16. Visser WA, Lee RA, Gielen MJ. Factors affecting the distribution of neural blockade by local anesthetics in epidural anesthesia and a comparison of lumbar versus thoracic epidural anesthesia. Anesth Analg. 2008; 107:708–721. PMID: 18633056.

17. Burstal R, Wegener F, Hayes C, Lantry G. Epidural analgesia: prospective audit of 1062 patients. Anaesth Intensive Care. 1998; 26:165–172. PMID: 9564395.

18. Wigfull J, Welchew E. Survey of 1057 patients receiving postoperative patient-controlled epidural analgesia. Anaesthesia. 2001; 56:70–75. PMID: 11167440.

19. Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001; 87:36–46. PMID: 11460812.

Fig. 2
Comparison between numerical pain scores according to the type of surgery at postoperative assessment intervals. The box plot displays 10th, 25th, median (fat bars), 75th, and 90th percentiles of values. PACU: post-anesthesia care unit. *P < 0.05 using the Friedman test with post-hoc testing using the Wilcoxon rank-sum test with Bonferroni correction.
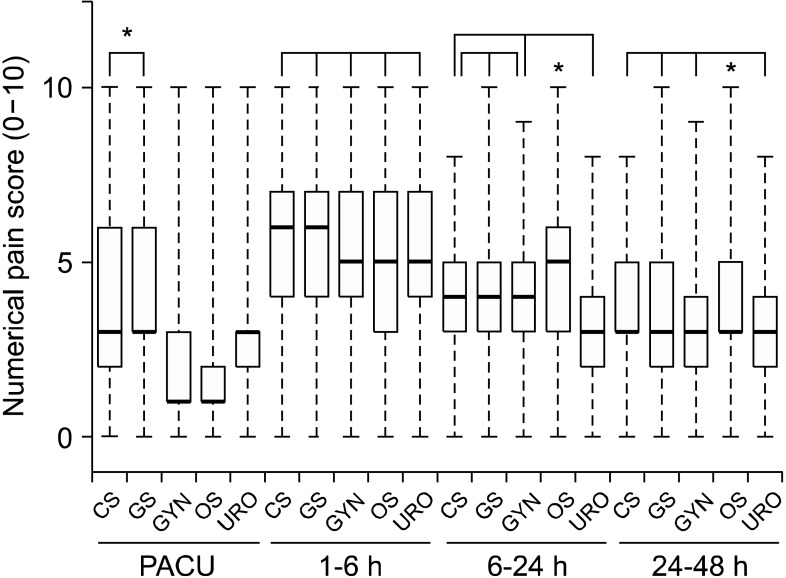
Fig. 3
Comparison between numerical pain scores according to the type of anesthesia (general vs. regional) at postoperative assessment intervals for each surgery type. The box plot displays 10th, 25th, median (fat bars), 75th, and 90th percentiles of values. PACU: post-anesthesia care unit. *P < 0.05 vs. regional anesthesia using the Friedman test.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download