Abstract
Smooth muscle antibodies (SMAs) are diagnostic markers for the serological diagnosis of type 1 autoimmune hepatitis. SMA that is restricted to staining of the stomach muscle and blood vessel walls was referred to as "SMA-V". In addition, SMAs are classified into the peritubular (SMA-T) and glomerular (SMA-G) patterns. SMAs are occasionally present in patients with malignancies, but have not yet been reported in thyroid cancer. We came across the first case of SMA positivity in a patient with papillary thyroid carcinoma (PTC). A 31-yr-old male was admitted to our hospital for evaluation of incidentally detected thyroid cancer. He had been diagnosed with PTC based on pathological results following fine-needle aspiration biopsy. The patient underwent total thyroidectomy followed by radio-iodine treatment. The serum levels of AST and ALT were increased before radiotherapy. Tests were conducted for the evaluation of liver disease. SMA was positive at a titer of 1:320, showing positive results for the vessel walls but negative results for the glomerulus and tubules in the kidney (SMA-V pattern). The association of SMA with malignancies and the classification of SMA immunofluorescent subtypes have been previously reported. However, these studies have not clearly established the ability of SMA subtype to predict a specific disease. Therefore, evaluation of an association of SMA pattern with specific diseases in SMA-positive patients may provide additional and useful information for the rapid diagnosis and accurate treatment of patients with autoimmune diseases or malignancies. This case report could serve as a great resource for further studies on SMA.
Figures and Tables
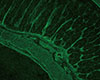
Fig. 1
Smooth muscle autoantibodies stain hepatic portal tracts with the walls of vessels in an indirect immunofluorescence assay of the mouse liver substrate (×200, fluorescein isothiocyanate).
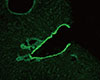
Fig. 2
Immunofluorescent pattern of smooth muscle autoantibodies (SMA) on a mouse stomach section. SMA stains muscularis mucosa, lamina propria and interglandular contractile fibers (×200, fluorescein isothiocyanate).
References
1. Liberal R, Mieli-Vergani G, Vergani D. Clinical significance of autoantibodies in autoimmune hepatitis. J Autoimmun. 2013; 46:17–24.

2. Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Diagnostic criteria of autoimmune hepatitis. Autoimmun Rev. 2014; 13:435–440.

3. Vergani D, Alvarez F, Bianchi FB, Cançado EL, Mackay IR, Manns MP, et al. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004; 41:677–683.

4. Bottazzo GF, Florin-Christensen A, Fairfax A, Swana G, Doniach D, Groeschel-Stewart U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol. 1976; 29:403–410.

5. Whitehouse JM, Holborow EJ. Smooth muscle antibody in malignant disease. Br Med J. 1971; 4:511–513.

6. Tannenberg AE, Muller HK, Cauchi MN, Nairn RC. Incidence of autoantibodies in cancer patients. Clin Exp Immunol. 1973; 15:153–156.
7. Toh BH. Smooth muscle autoantibodies and autoantigens. Clin Exp Immunol. 1979; 38:621–628.
8. Lidman K, Biberfeld G, Fagraeus A, Norberg R, Torstensson R, Utter G, et al. Anti-actin specificity of human smooth muscle antibodies in chronic active hepatitis. Clin Exp Immunol. 1976; 24:266–272.
9. Granito A, Muratori L, Muratori P, Pappas G, Guidi M, Cassani F, et al. Antibodies to filamentous actin (F-actin) in type 1 autoimmune hepatitis. J Clin Pathol. 2006; 59:280–284.

10. Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000; 43:1431–1442.

11. Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996; 25:283–291.

12. Rönnblom LE, Alm GV, Oberg K. Autoimmune phenomena in patients with malignant carcinoid tumors during interferon-alpha treatment. Acta Oncol. 1991; 30:537–540.

13. Holbro A, Abinun M, Daikeler T. Management of autoimmune diseases after haematopoietic stem cell transplantation. Br J Haematol. 2012; 157:281–290.

14. Aubert O, Sberro-Soussan R, Scemla A, Casadevall N, Teyssandier I, Martinez F, et al. Autoimmune neutropenia after kidney transplantation: a disregarded entity of posttransplant neutropenia. Transplantation. 2014; 97:725–729.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download