1. Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP, Douglas KM, Kitas GD. Platelet function in rheumatoid arthritis: arthritic and cardiovascular implications. Rheumatol Int. 2011; 31:153–164. PMID:
20390282.
2. Olumuyiwa-Akeredolu OO, Pretorius E. Rheumatoid arthritis: notable biomarkers linking to chronic systemic conditions and cancer. Curr Pharm Des. 2016; 22:918–924. PMID:
26648464.
3. Scherlinger M, Guillotin V, Truchetet ME, Contin-Bordes C, Sisirak V, Duffau P, et al. Systemic lupus erythematosus and systemic sclerosis: all roads lead to platelets. Autoimmun Rev. 2018; 17:625–635. PMID:
29635077.
4. Gasparyan AY, Sandoo A, Stavropoulos-Kalinoglou A, Kitas GD. Mean platelet volume in patients with rheumatoid arthritis: the effect of anti-TNF-α therapy. Rheumatol Int. 2010; 30:1125–1129. PMID:
20066426.
5. Abdel Galil SM, Edrees AM, Ajeeb AK, Aldoobi GS, El-Boshy M, Hussain W. Prognostic significance of platelet count in SLE patients. Platelets. 2017; 28:203–207. PMID:
27590999.
6. Lood C, Tydén H, Gullstrand B, Nielsen CT, Heegaard NHH, Linge P, et al. Decreased platelet size is associated with platelet activation and anti-phospholipid syndrome in systemic lupus erythematosus. Rheumatology (Oxford). 2017; 56:408–416. PMID:
28031442.
7. Milovanovic M, Nilsson E, Järemo P. Relationships between platelets and inflammatory markers in rheumatoid arthritis. Clin Chim Acta. 2004; 343:237–240. PMID:
15115702.
8. Zha Q, He Y, Lu Y, Lu A. Relationship between platelet counts and cartilage erosion in 436 cases of rheumatoid arthritis. Clin Chim Acta. 2006; 371:194–195. PMID:
16626675.
9. Matsuno H. Remarkable efficacy of tocilizumab for treating rheumatoid arthritis in patients with high platelet counts. Mod Rheumatol. 2015; 25:38–42. PMID:
25529071.
10. Üsküdar Cansu D, Üsküdar Teke H, Musmul A, Korkmaz C. Is thrombocytosis always an indicator of autosplenectomy in patients with systemic lupus erythematosus. Rheumatol Int. 2018; 38:239–247. PMID:
29119304.
11. Li J, Pan Z, Liu H, Ding F, Shu Q, Li X. Retrospective analysis of the risk of hemorrhage associated with moderate and severe thrombocytopenia of 173 patients with systemic lupus erythematosus. Medicine (Baltimore). 2018; 97:e11356. PMID:
29979417.
12. El-Dairi MA, Chang L, Proia AD, Cummings TJ, Stinnett SS, Bhatti MT. Diagnostic algorithm for patients with suspected giant cell arteritis. J Neuroophthalmol. 2015; 35:246–253. PMID:
25802967.
13. Bornstein G, Barshack I, Koren-Morag N, Ben-Zvi I, Furie N, Grossman C. Negative temporal artery biopsy: predictive factors for giant cell arteritis diagnosis and alternate diagnoses of patients without arteritis. Clin Rheumatol. 2018; 37:2819–2824. PMID:
29549493.
14. Han JW, Oh JH, Rhim JW, Lee KY. Correlation between elevated platelet count and immunoglobulin levels in the early convalescent stage of Kawasaki disease. Medicine (Baltimore). 2017; 96:e7583. PMID:
28723797.
15. Maric LS, Knezovic I, Papic N, Mise B, Roglic S, Markovinovic L, et al. Risk factors for coronary artery abnormalities in children with Kawasaki disease: a 10-year experience. Rheumatol Int. 2015; 35:1053–1058. PMID:
25429794.
16. Elmas AT. Platelet counts in children with Henoch-Schonlein purpura—relationship to renal involvement. J Clin Lab Anal. 2016; 30:71–74. PMID:
25385472.
17. Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogné M, Richard Y, et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007; 35:1376–1387. PMID:
17656005.
18. Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018; 122:337–351. PMID:
29348254.
19. Yeung J, Li W, Holinstat M. Platelet signaling and disease: targeted therapy for thrombosis and other related diseases. Pharmacol Rev. 2018; 70:526–548. PMID:
29925522.
20. Olumuyiwa-Akeredolu OO, Pretorius E. Platelet and red blood cell interactions and their role in rheumatoid arthritis. Rheumatol Int. 2015; 35:1955–1964. PMID:
26059943.
21. Łukasik ZM, Makowski M, Makowska JS. From blood coagulation to innate and adaptive immunity: the role of platelets in the physiology and pathology of autoimmune disorders. Rheumatol Int. 2018; 38:959–974. PMID:
29492586.
22. Zamora C, Cantó E, Nieto JC, Bardina J, Diaz-Torné C, Moya P, et al. Binding of platelets to lymphocytes: a potential anti-inflammatory therapy in rheumatoid arthritis. J Immunol. 2017; 198:3099–3108. PMID:
28250158.
23. Hally KE, La Flamme AC, Harding SA, Larsen PD. Platelets regulate leucocyte responses to Toll-like receptor stimulation. Clin Transl Immunology. 2018; 7:e1036. PMID:
30065836.
24. Duchez AC, Boudreau LH, Naika GS, Bollinger J, Belleannée C, Cloutier N, et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc Natl Acad Sci U S A. 2015; 112:E3564–E3573. PMID:
26106157.
25. Bunescu A, Seideman P, Lenkei R, Levin K, Egberg N. Enhanced Fcgamma receptor I. alphaMbeta2 integrin receptor expression by monocytes and neutrophils in rheumatoid arthritis: interaction with platelets. J Rheumatol. 2004; 31:2347–2355. PMID:
15570633.
26. Manfredi AA, Baldini M, Camera M, Baldissera E, Brambilla M, Peretti G, et al. Anti-TNFα agents curb platelet activation in patients with rheumatoid arthritis. Ann Rheum Dis. 2016; 75:1511–1520. PMID:
26819099.
27. Kornerup KN, Salmon GP, Pitchford SC, Liu WL, Page CP. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol (1985). 2010; 109:758–767. PMID:
20558756.
28. Maugeri N, Rovere-Querini P, Evangelista V, Godino C, Demetrio M, Baldini M, et al. An intense and short-lasting burst of neutrophil activation differentiates early acute myocardial infarction from systemic inflammatory syndromes. PLoS One. 2012; 7:e39484. PMID:
22761804.
29. Habets KL, Trouw LA, Levarht EW, Korporaal SJ, Habets PA, de Groot P, et al. Anti-citrullinated protein antibodies contribute to platelet activation in rheumatoid arthritis. Arthritis Res Ther. 2015; 17:209. PMID:
26268317.
30. Lam FW, Da Q, Guillory B, Cruz MA. Recombinant human vimentin binds to p-selectin and blocks neutrophil capture and rolling on platelets and endothelium. J Immunol. 2018; 200:1718–1726. PMID:
29335256.
31. Cheng Q, Hoi A, Hickey MJ, Morand EF. Lymphocytes from systemic lupus erythematosus patients display increased spreading on VCAM-1, an effect associated with active renal involvement. Lupus. 2012; 21:632–641. PMID:
22345121.
32. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010; 6:149–163. PMID:
20021215.
33. Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013; 8:e67888. PMID:
23874462.
34. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet: lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014; 23:1204–1212. PMID:
24793958.
35. Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet: lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2016; 95:e3837. PMID:
27310960.
36. Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep. 2017; 7:40426. PMID:
28071752.
37. Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int Angiol. 2018; 37:4–11. PMID:
28541022.
38. Mischler K, Fischer JE, Zgraggen L, Kudielka BM, Preckel D, von Känel R. The effect of repeated acute mental stress on habituation and recovery responses in hemoconcentration and blood cells in healthy men. Life Sci. 2005; 77:1166–1179. PMID:
15978266.
39. Aktar F, Tekin R. Mean platelet volume, neutrophil to lymphocyte ratio and platelet: lymphocyte ratio in determining the diagnosis or outcome in children with snakebite. Arch Argent Pediatr. 2017; 115:576–580. PMID:
29087117.
40. Kilincalp S, Çoban Ş, Akinci H, Hamamcı M, Karaahmet F, Coşkun Y, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2015; 24:328–333. PMID:
25304028.
41. Ozawa T, Ishihara S, Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, et al. The preoperative platelet to lymphocyte ratio is a prognostic marker in patients with stage II colorectal cancer. Int J Colorectal Dis. 2015; 30:1165–1171. PMID:
26049902.
42. Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012; 17:216–222. PMID:
22424597.
43. Kim JH, Lee JY, Kim HK, Lee JW, Jung SG, Jung K, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol. 2017; 23:505–515. PMID:
28210087.
44. You J, Zhang H, Shen Y, Chen C, Liu W, Zheng M, et al. Impact of platelet to lymphocyte ratio and metabolic syndrome on the prognosis of colorectal cancer patients. Onco Targets Ther. 2017; 10:2199–2208. PMID:
28458563.
45. Akboga MK, Canpolat U, Yayla C, Ozcan F, Ozeke O, Topaloglu S, et al. Association of platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology. 2016; 67:89–95. PMID:
25922197.
46. Akboga MK, Canpolat U, Yuksel M, Yayla C, Yilmaz S, Turak O, et al. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: a single center large-scale study. Platelets. 2016; 27:178–183. PMID:
26196312.
47. Li H, Zhou Y, Ma Y, Han S, Zhou L. The prognostic value of the platelet: lymphocyte ratio in acute coronary syndrome: a systematic review and meta-analysis. Kardiol Pol. 2017; 75:666–673. PMID:
28394006.
48. Akboga YE, Bektas H, Anlar O. Usefulness of platelet to lymphocyte and neutrophil to lymphocyte ratios in predicting the presence of cerebral venous sinus thrombosis and in-hospital major adverse cerebral events. J Neurol Sci. 2017; 380:226–229. PMID:
28870575.
49. Artoni A, Abbattista M, Bucciarelli P, Gianniello F, Scalambrino E, Pappalardo E, et al. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio as risk factors for venous thrombosis. Clin Appl Thromb Hemost. 2018; 24:808–814. PMID:
29088921.
50. Yang W, Liu Y. Platelet-lymphocyte ratio is a predictor of venous thromboembolism in cancer patients. Thromb Res. 2015; 136:212–215. PMID:
25533129.
51. Tham T, Rahman L, Persaud C, Olson C, Costantino P. Venous thromboembolism risk in head and neck cancer: significance of the preoperative platelet-to-lymphocyte ratio. Otolaryngol Head Neck Surg. 2018; 159:85–91. PMID:
29406795.
52. Koseoglu SB. Bone loss and platelet-to-lymphocyte ratio. Biomark Med. 2017; 11:5–10. PMID:
27917655.
53. Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011; 31:1409–1417. PMID:
21800117.
54. Peng YF, Cao L, Zeng YH, Zhang ZX, Chen D, Zhang Q, et al. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in patients with rheumatoid arthritis. Open Med (Wars). 2015; 10:249–253. PMID:
28352702.
55. Fu H, Qin B, Hu Z, Ma N, Yang M, Wei T, et al. Neutrophil- and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin Lab. 2015; 61:269–273. PMID:
25974992.
56. Uslu AU, Küçük A, Şahin A, Ugan Y, Yılmaz R, Güngör T, et al. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis. 2015; 18:731–735. PMID:
25900081.
57. Zhang Y, Yin Y, Kuai S, Shan Z, Pei H, Wang J. Combination of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic biomarker for rheumatoid arthritis. Int J Clin Exp Med. 2016; 9:22076–22081.
58. Wu Y, Chen Y, Yang X, Chen L, Yang Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol. 2016; 36:94–99. PMID:
27111516.
59. Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, et al. Neutrophil to lymphocyte ratio (NLR) and platelet: lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016; 26:372–376. PMID:
26403379.
60. Kim HA, Jung JY, Suh CH. Usefulness of neutrophil-to-lymphocyte ratio as a biomarker for diagnosing infections in patients with systemic lupus erythematosus. Clin Rheumatol. 2017; 36:2479–2485. PMID:
28840341.
61. Huang Y, Deng W, Zheng S, Feng F, Huang Z, Huang Q, et al. Relationship between monocytes to lymphocytes ratio and axial spondyloarthritis. Int Immunopharmacol. 2018; 57:43–46. PMID:
29471252.
62. Bozan N, Alpaycı M, Aslan M, Cankaya H, Kıroglu AF, Turan M, et al. Mean platelet volume, red cell distribution width, platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios in patients with ankylosing spondylitis and their relationships with high-frequency hearing thresholds. Eur Arch Otorhinolaryngol. 2016; 273:3663–3672. PMID:
27034281.
63. Seng JJB, Kwan YH, Low LL, Thumboo J, Fong WSW. Role of neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and mean platelet volume (MPV) in assessing disease control in Asian patients with axial spondyloarthritis. Biomarkers. 2018; 23:335–338. PMID:
29307233.
64. Kim DS, Shin D, Lee MS, Kim HJ, Kim DY, Kim SM, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016; 43:305–310. PMID:
26381893.
65. Alan S, Tuna S, Türkoğlu EB. The relation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume with the presence and severity of Behçet's syndrome. Kaohsiung J Med Sci. 2015; 31:626–631. PMID:
26709224.
66. Jiang Y, Zang M, Li S. Serum PLR and LMR in Behçet's disease: can they show the disease activity? Medicine (Baltimore). 2017; 96:e6981. PMID:
28538403.
67. Avci A, Avci D, Erden F, Ragip E, Cetinkaya A, Ozyurt K, et al. Can we use the neutrophil: lymphocyte ratio, platelet: lymphocyte ratio, and mean platelet volume values for the diagnosis of anterior uveitis in patients with Behcet's disease? Ther Clin Risk Manag. 2017; 13:881–886. PMID:
28769565.
68. La Regina M, Gasparyan AY, Orlandini F, Prisco D. Behçet's disease as a model of venous thrombosis. Open Cardiovasc Med J. 2010; 4:71–77. PMID:
20360979.
69. Cocco G, Gasparyan AY. Behçet's disease: an insight from a cardiologist's point of view. Open Cardiovasc Med J. 2010; 4:63–70. PMID:
20360978.
70. Balkarli A, Kucuk A, Babur H, Erbasan F. Neutrophil/lymphocyte ratio and mean platelet volume in Behçet's disease. Eur Rev Med Pharmacol Sci. 2016; 20:3045–3050. PMID:
27460734.
71. Oh LJ, Wong E, Andrici J, McCluskey P, Smith JEH, Gill AJ. Full blood count as an ancillary test to support the diagnosis of giant cell arteritis. Intern Med J. 2018; 48:408–413. PMID:
29236347.
72. Walvick MD, Walvick MP. Giant cell arteritis: laboratory predictors of a positive temporal artery biopsy. Ophthalmology. 2011; 118:1201–1204. PMID:
21232803.
73. Pan L, Du J, Li T, Liao H. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio associated with disease activity in patients with Takayasu's arteritis: a case-control study. BMJ Open. 2017; 7:e014451.
74. Park HJ, Jung SM, Song JJ, Park YB, Lee SW. Platelet to lymphocyte ratio is associated with the current activity of ANCA-associated vasculitis at diagnosis: a retrospective monocentric study. Rheumatol Int. 2018; 38:1865–1871. PMID:
30088046.
75. Shin J, Lee H, Eun L. Verification of current risk scores for Kawasaki disease in Korean children. J Korean Med Sci. 2017; 32:1991–1996. PMID:
29115081.
76. Ha KS, Jang GY, Lee J, Lee KC, Son CS. Laboratory markers in incomplete Kawasaki disease according to coronary artery outcome. Korean Circ J. 2018; 48:287–295. PMID:
29625511.
77. Kawamura Y, Takeshita S, Kanai T, Yoshida Y, Nonoyama S. The combined usefulness of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in predicting intravenous immunoglobulin resistance with Kawasaki disease. J Pediatr. 2016; 178:281.e1–284.e1. PMID:
27526622.
78. Takeshita S, Kanai T, Kawamura Y, Yoshida Y, Nonoyama S. A comparison of the predictive validity of the combination of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio and other risk scoring systems for intravenous immunoglobulin (ivig)-resistance in Kawasaki disease. PLoS One. 2017; 12:e0176957. PMID:
28542183.
79. Yuan YD, Sun J, Li PF, Wei CL, Yu YH. Values of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in predicting sensitivity to intravenous immunoglobulin in Kawasaki disease. Zhongguo Dang Dai Er Ke Za Zhi. 2017; 19:410–413. PMID:
28407827.
80. Chantasiriwan N, Silvilairat S, Makonkawkeyoon K, Pongprot Y, Sittiwangkul R. Predictors of intravenous immunoglobulin resistance and coronary artery aneurysm in patients with Kawasaki disease. Paediatr Int Child Health. 2018; 38:209–212. PMID:
29768976.
81. Özer S, Yılmaz R, Sönmezgöz E, Karaaslan E, Taşkın S, Bütün İ, et al. Simple markers for subclinical inflammation in patients with familial Mediterranean fever. Med Sci Monit. 2015; 21:298–303. PMID:
25615955.
82. Kelesoglu FM, Aygun E, Okumus NK, Ersoy A, Karapınar E, Saglam N, et al. Evaluation of subclinical inflammation in familial Mediterranean fever patients: relations with mutation types and attack status: a retrospective study. Clin Rheumatol. 2016; 35:2757–2763. PMID:
27106545.
83. Ozçakar ZB, Yalçınkaya F. Vascular comorbidities in familial Mediterranean fever. Rheumatol Int. 2011; 31:1275–1281. PMID:
21437693.
84. Tatsi C, Boden R, Sinaii N, Keil M, Lyssikatos C, Belyavskaya E, et al. Decreased lymphocytes and increased risk for infection are common in endogenous pediatric Cushing syndrome. Pediatr Res. 2018; 83:431–437. PMID:
29211058.
85. Bromberg L, Roufosse F, Pradier O, Delporte C, Van Antwerpen P, De Maertelaer V, et al. Methylprednisolone-induced lymphocytosis in patients with immune-mediated inflammatory disorders. Am J Med. 2016; 129:746–752. PMID:
26968468.
86. Song T, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ. Single-dose versus two-dose administration of methotrexate for the treatment of ectopic pregnancy: a randomized controlled trial. Hum Reprod. 2016; 31:332–338. PMID:
26701971.
87. Harigai M. Lymphoproliferative disorders in patients with rheumatoid arthritis in the era of widespread use of methotrexate: a review of the literature and current perspective. Mod Rheumatol. 2018; 28:1–8. PMID:
28758827.
88. Inui Y, Matsuoka H, Yakushijin K, Okamura A, Shimada T, Yano S, et al. Methotrexate-associated lymphoproliferative disorders: management by watchful waiting and observation of early lymphocyte recovery after methotrexate withdrawal. Leuk Lymphoma. 2015; 56:3045–3051. PMID:
25721751.
89. Takanashi S, Aisa Y, Ito C, Arakaki H, Osada Y, Amano Y, et al. Clinical characteristics of methotrexate-associated lymphoproliferative disorders: relationship between absolute lymphocyte count recovery and spontaneous regression. Rheumatol Int. 2017; 37:1629–1633. PMID:
28676912.
90. Sargin G, Senturk T, Yavasoglu I, Kose R. Relationship between neutrophil-lymphocyte, platelet : lymphocyte ratio and disease activity in rheumatoid arthritis treated with rituximab. Int J Rheum Dis. 2018; 21:2122–2127. PMID:
30338636.
91. Asahina A, Kubo N, Umezawa Y, Honda H, Yanaba K, Nakagawa H. Neutrophil-lymphocyte ratio, platelet: lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: response to therapy with biologics. J Dermatol. 2017; 44:1112–1121. PMID:
28493493.
92. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011; 17:47–58. PMID:
21247392.
93. Uslu AU, Inal S, Balta S. Assessment of platelet-lymphocyte ratio based on EDTA-dependent pseudothrombocytopenia. Angiology. 2016; 67:96–97. PMID:
26124494.
94. Ohashi-Fukuda N, Inokuchi R, Sato H, Nakamura K, Iwagami M, Wada T, et al. Poorer prognosis with ethylenediaminetetraacetic acid-dependent pseudothrombocytopenia: a single-center case-control study. Medicine (Baltimore). 2015; 94:e674. PMID:
25881844.
95. Kweon OJ, Lee MK, Kim HJ, Chung JW, Choi SH, Kim HR. Neutropenia and neutrophil : lymphocyte ratio in a healthy Korean population: race and sex should be considered. Int J Lab Hematol. 2016; 38:308–318. PMID:
27018397.
96. Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore). 2018; 97:e11138. PMID:
29952958.
97. Nah EH, Kim S, Cho S, Cho HI. Complete blood count reference intervals and patterns of changes across pediatric, adult, and geriatric ages in Korea. Ann Lab Med. 2018; 38:503–511. PMID:
30027692.
98. Crawford VL, McNerlan SE, Stout RW. Seasonal changes in platelets, fibrinogen and factor VII in elderly people. Age Ageing. 2003; 32:661–665. PMID:
14600009.
99. Noris P, Melazzini F, Balduini CL. New roles for mean platelet volume measurement in the clinical practice? Platelets. 2016; 27:607–612. PMID:
27686008.
100. Kocak MZ, Aktas G, Erkus E, Duman TT, Atak BM, Savli H. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018; 28:844–847. PMID:
30369376.
101. Gogoi P, Sinha P, Gupta B, Firmal P, Rajaram S. Neutrophil-to-lymphocyte ratio and platelet indices in pre-eclampsia. Int J Gynaecol Obstet. 2019; 144:16–20. PMID:
30362112.
102. Sağ S, Sağ MS, Tekeoğlu I, Kamanlı A, Nas K, Acar BA. Relationship of hematologic markers with IL-17 and IL-1 beta in patients with rheumatoid arthritis. J Back Musculoskelet Rehabil. 2018; 31:703–707. PMID:
29578474.
103. Tekeoğlu İ, Gürol G, Harman H, Karakeçe E, Çiftçi İH. Overlooked hematological markers of disease activity in rheumatoid arthritis. Int J Rheum Dis. 2016; 19:1078–1082. PMID:
26620362.
104. Xie S, Chen X. Red blood cell distribution width-to-platelet ratio as a disease activity-associated factor in systemic lupus erythematosus. Medicine (Baltimore). 2018; 97:e12342. PMID:
30278511.
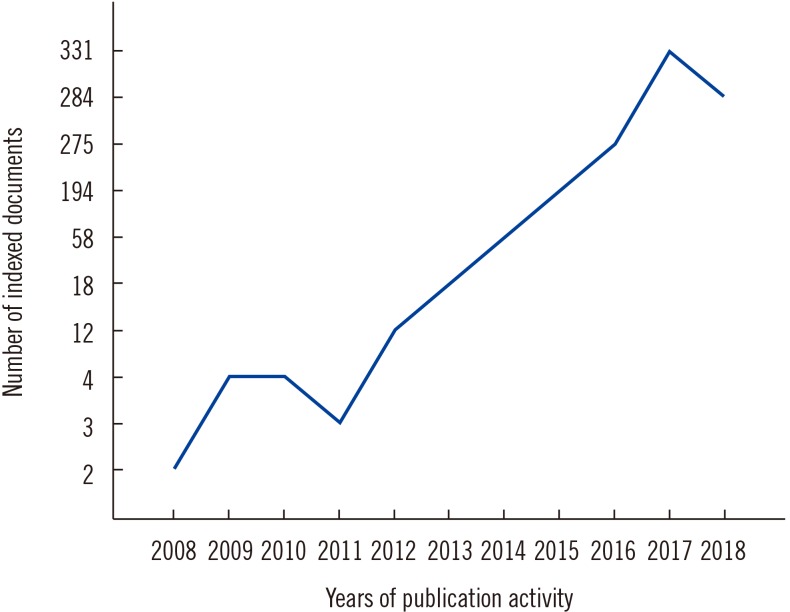
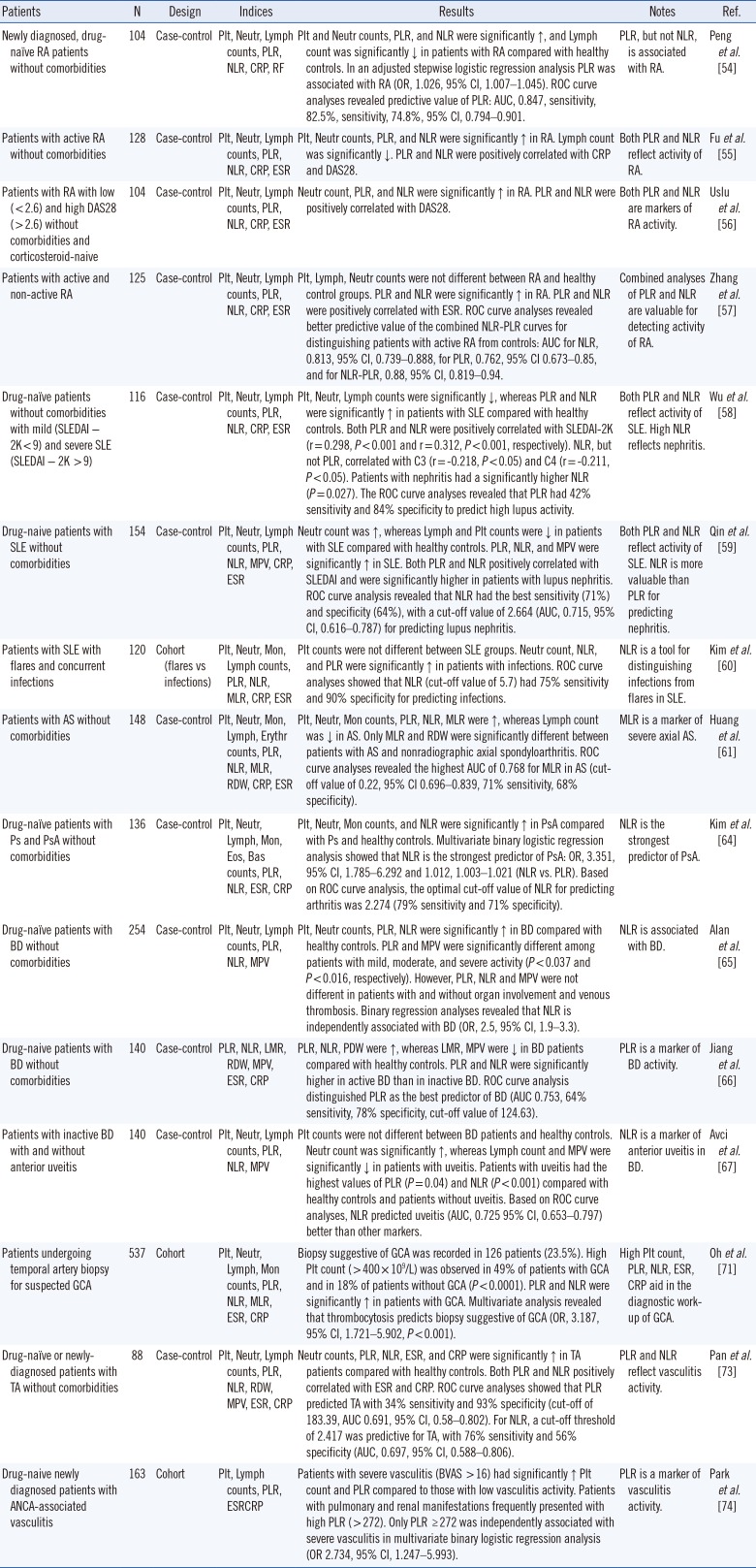
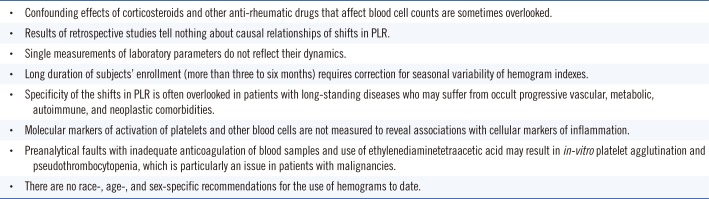




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download