1. Jakobsson B, Esbjörner E, Hansson S. Minimum incidence and diagnostic rate of first urinary tract infection. Pediatrics. 1999; 104:222–226. PMID:
10428998.
2. Peters CA, Skoog SJ, Arant BS Jr, Copp HL, Elder JS, Hudson RG, et al. Summary of the AUA guideline on management of primary vesicoureteral reflux in children. J Urol. 2010; 184:1134–1144. PMID:
20650499.
3. Park YS. Renal scar formation after urinary tract infection in children. Korean J Pediatr. 2012; 55:367–370. PMID:
23133482.
4. Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J. 1997; 16:735–746. PMID:
9271034.
5. de Man P. Bacterial attachment, inflammation and renal scarring in urinary tract infection. Wien Med Wochenschr. 1991; 141:537–540. PMID:
1810092.
6. Mori R, Yonemoto N, Fitzgerald A, Tullus K, Verrier-Jones K, Lakhanpaul M. Diagnostic performance of urine dipstick testing in children with suspected UTI: a systematic review of relationship with age and comparison with microscopy. Acta Paediatr. 2010; 99:581–584. PMID:
20055779.
7. Goldraich NP, Goldraich IH. Update on dimercaptosuccinic acid renal scanning in children with urinary tract infection. Pediatr Nephrol. 1995; 9:221–226. PMID:
7794724.
8. Lee HE, Kim DK, Kang HK, Park K. The diagnosis of febrile urinary tract infection in children may be facilitated by urinary biomarkers. Pediatr Nephrol. 2015; 30:123–130. PMID:
25127917.
9. Forster CS, Devarajan P. Neutrophil gelatinase-associated lipocalin: utility in urologic conditions. Pediatr Nephrol. 2017; 32:377–381. PMID:
27785626.
10. Yim HE, Yim H, Bae ES, Woo SU, Yoo KH. Predictive value of urinary and serum biomarkers in young children with febrile urinary tract infections. Pediatr Nephrol. 2014; 29:2181–2189. PMID:
24924751.
11. Sim JH, Yim HE, Choi BM, Lee JH, Yoo KH. Plasma neutrophil gelatinase-associated lipocalin predicts acute pyelonephritis in children with urinary tract infections. Pediatr Res. 2015; 78:48–55. PMID:
25790277.
12. Valdimarsson S, Jodal U, Barregård L, Hansson S. Urine neutrophil gelatinase-associated lipocalin and other biomarkers in infants with urinary tract infection and in febrile controls. Pediatr Nephrol. 2017; 32:2079–2087. PMID:
28756475.
13. Seo WH, Nam SW, Lee EH, Je BK, Yim HE, Choi BM. A rapid plasma neutrophil gelatinase-associated lipocalin assay for diagnosis of acute pyelonephritis in infants with acute febrile urinary tract infections: a preliminary study. Eur J Pediatr. 2014; 173:229–232. PMID:
23918295.
14. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011; 128:595–610. PMID:
21873693.
15. International Reflux Study Committee. Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics. 1981; 67:392–400. PMID:
7017578.
16. Elder JS. Urinary tract infections. In : Kliegman RM, editor. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders;2011. p. 1829–1834.
17. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009; 54:1012–1024. PMID:
19850388.
18. Kim BH, Yu N, Kim HR, Yun KW, Lim IS, Kim TH, et al. Evaluation of the optimal neutrophil gelatinase-associated lipocalin value as a screening biomarker for urinary tract infections in children. Ann Lab Med. 2014; 34:354–359. PMID:
25187887.
19. Kim BK, Yim HE, Yoo KH. Plasma neutrophil gelatinase-associated lipocalin: a marker of acute pyelonephritis in children. Pediatr Nephrol. 2017; 32:477–484. PMID:
27744618.
20. Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012; 50:1505–1517. PMID:
22962216.
21. Stejskal D, Karpísek M, Humenanska V, Hanulova Z, Stejskal P, Kusnierova P, et al. Lipocalin-2: development, analytical characterization, and clinical testing of a new ELISA. Horm Metab Res. 2008; 40:381–385. PMID:
18393169.
22. Di Somma S, Magrini L, De Berardinis B, Marino R, Ferri E, Moscatelli P, et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013; 17:R29. PMID:
23402494.
23. Cangemi G, Storti S, Cantinotti M, Fortunato A, Emdin M, Bruschettini M, et al. Reference values for urinary neutrophil gelatinase-associated lipocalin (NGAL) in pediatric age measured with a fully automated chemiluminescent platform. Clin Chem Lab Med. 2013; 51:1101–1105. PMID:
23183760.
24. Fjaertoft G, Foucard T, Xu S, Venge P. Human neutrophil lipocalin (HNL) as a diagnostic tool in children with acute infections: a study of the kinetics. Acta Paediatr. 2005; 94:661–666. PMID:
16188765.
25. Rafiei A, Mohammadjafari H, Bazi S, Mirabi AM. Urinary neutrophil gelatinase-associated lipocalin (NGAL) might be an independent marker for anticipating scar formation in children with acute pyelonephritis. J Renal Inj Prev. 2015; 4:39–44. PMID:
26060836.
26. Parmaksiz G, Noyan A, Dursun H, Ince E, Anarat R, Cengiz N. Role of new biomarkers for predicting renal scarring in vesicoureteral reflux: NGAL, KIM-1, and L-FABP. Pediatr Nephrol. 2016; 31:97–103. PMID:
26324091.
27. Noyan A, Parmaksiz G, Dursun H, Ezer SS, Anarat R, Cengiz N. Urinary NGAL, KIM-1 and L-FABP concentrations in antenatal hydronephrosis. J Pediatr Urol. 2015; 11:249.e1–249.e6. PMID:
26096437.
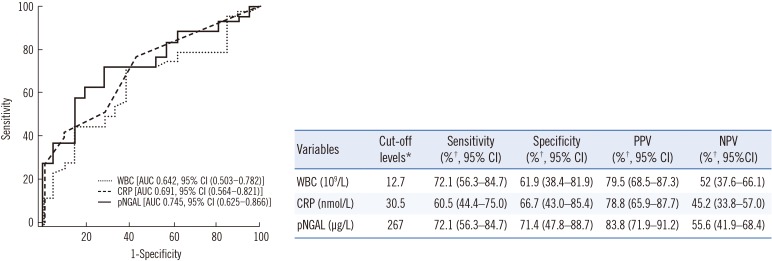
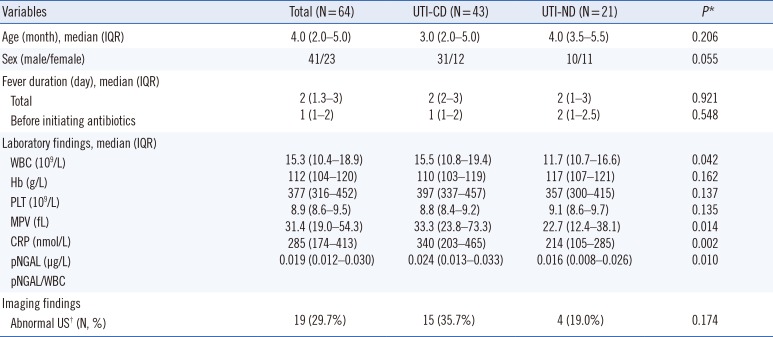
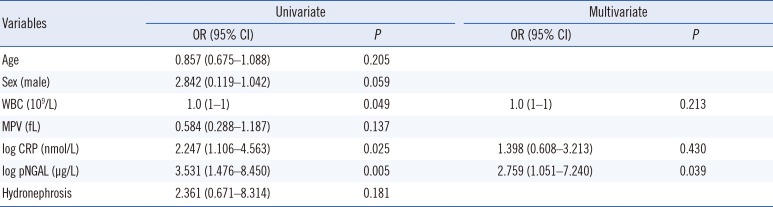




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download