Dear Editor,
Although therapy-related acute leukemia (tAL) is a well-recognized clinical syndrome and is increasing owing to the prolonged survival of patients treated with chemoradiotherapy [1], tAL with mixed phenotype is extremely rare. Only four cases of tAL with mixed phenotype have been reported worldwide; among them, only one case occurred after hematologic malignancy [23]. Here, we report a rare case of tAL with mixed phenotype with BCR-ABL1 after achieving complete remission (CR) of diffuse large B-cell lymphoma (DLBCL).
A 57-yr-old woman with Hashimoto’s disease was admitted with chronic epigastric discomfort in August 2009. She was diagnosed as having non-Hodgkin lymphoma with supraclavicular lymph node metastasis (stage IV). There was no evidence of lymphoma involvement or chromosomal abnormalities on bone marrow examination. She received rituximab, cyclophosphamide, vincristine, prednisone and achieved CR. During follow-up in October 2012, endoscopy revealed a 4-cm round, infiltrating mass on the duodenal bulb. A biopsy revealed DLBCL. The patient received rituximab, cyclophosphamide, vincristine, hydroxydaunorubicin, prednisone with granulocyte colony-stimulating factor (G-CSF) injected after each cycle and achieved CR.
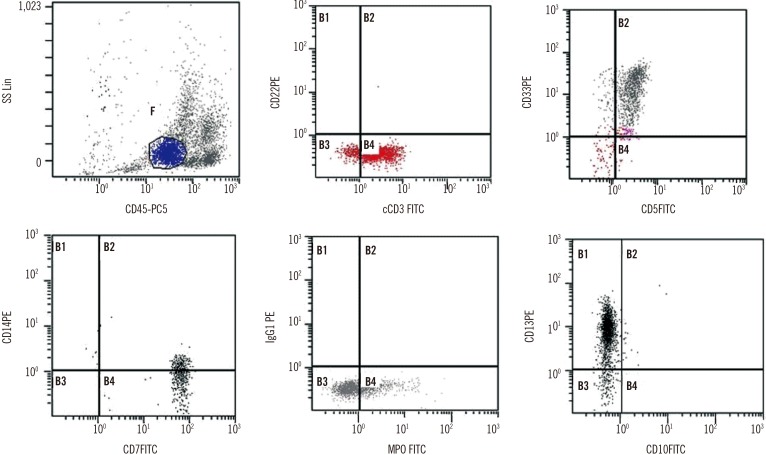
In August 2015, the patient was readmitted to the hospital after a follow-up examination revealed the presence of immature cells in the blood. Physical examination showed no specific findings, and complete blood count findings were as follows: hematocrit, 35.1%; hemoglobin, 116 g/L; platelet count, 129×109/L; and white blood cell count, 2.41×109/L, with 4% blasts, 26% segmented neutrophils, 3% band neutrophils, 39% lymphocytes, and 26% monocytes. Bone marrow aspirate smears revealed 40.7% leukemic blasts with medium cell size, oval to round shape, vesicular nuclei, fine chromatin patterns, and basophilic cytoplasm (Fig. 1A). On cytochemical staining, blasts were negative for Periodic acid–Schiff and non-specific esterase staining, but were positive for myeloperoxidase staining (Fig. 1B). Flow cytometric analysis showed that the blasts were positive for both T-lymphoid and myeloid markers (cytoplasmic CD3, 87%; CD5, 90%; CD7, 96%; cytoplasmic myeloperoxidase, 20%; CD13, 91%; CD33, 87%; Fig. 2) and negative for CD2, CD10, CD11b, CD14, CD15, CD19, CD20, CD61, CD117, and terminal deoxynucleotidyl transferase. Immunophenotyping fulfilled the diagnostic criteria of T/myeloid biphenotypic leukemia based on the scoring system of the European Group for the Immunological Characterization of Leukemias and WHO classifications [45]. FISH using the locus specific identifier BCR/ABL dual-color, dual-fusion translocation probe set (Abbott Molecular/Vysis, Des Plaines, IL, USA) was negative, but multiplex reverse transcription PCR (mRT-PCR) using HemaVision kit (Bio-Rad Laboratories, Hercules, CA, USA) revealed the presence of minor BCR-ABL1 (e1a2) fusion transcripts. False-positive results were minimized by using a previously diagnosed BCR-ABL1-positive patient sample as a positive control and normal patient sample as a negative control. As some reports claim that mRT-PCR is more sensitive than FISH in detecting cryptic translocations or submicroscopic insertion that are incapable of generating signals large enough to be visualized [6], the result may be due to submicroscopic translocation. Chromosome analysis of bone marrow cells failed because of insufficient mitotic cells. Immunoglobulin heavy chain gene rearrangement and T cell receptor-γ gene rearrangement were not detected on bone marrow aspirates.
The patient was diagnosed as having tAL with T/myeloid phenotype and minor BCR-ABL1 according to the 2008 WHO classification. She refused chemotherapy and succumbed in December 2015.
Mixed phenotype acute leukemia (MPAL) is an uncommon subtype that comprises 0.5-1% of leukemia. The T/myeloid phenotype is rarer and represents 35% of all MPAL cases [7]. Philadelphia chromosome is the most frequent chromosomal abnormality, accounting for approximately 30% of MPAL cases [8].
The risk of secondary malignancies after lymphoma treatment is relatively increased for leukemia. AML, ALL, MDS, CML and chronic myelomonocytic leukemia are reported secondary hematologic malignancies [19]. Until now, only one case of tAL with mixed phenotype after lymphoma has been reported [3]. To the best of our knowledge, this is the second case of tAL with mixed phenotype after DLBCL. This case is also unique because the BCR-ABL1 has not been described in the literature for patients with tAL with mixed phenotype, after hematologic malignancy.
According to the 2008 WHO classification, tAL can be attributed to radiation, alkylating agents, or topoisomerase II inhibitors [4]. Our patient did not receive radiation therapy but previously received cyclophosphamide and doxorubicin. Furthermore, some investigators have suggested that G-CSF stimulates proliferation of not only myeloid lineage cells but also some leukemic blast cells, including those of biphenotypic leukemia [10]. Thus, G-CSF injection might have played an additional role in this patient.
Therefore, this is the first case of tAL with mixed phenotype and BCR-ABL 1 after alkylating agent and topoisomerase II inhibitor therapy for DLBCL.
References
1. Leone G, Fianchi L, Voso MT. Therapy-related myeloid neoplasms. Curr Opin Oncol. 2011; 23:672–680. PMID: 21918440.
2. Cho JH, Hur M, Moon HW, Yun YM, Ko YS, Kim WS, et al. Therapy-related acute leukemia with mixed phenotype and t(9;22)(q32;q11.2): a case report and review of the literature. Hum Pathol. 2012; 43:605–609. PMID: 22036054.
3. Roberts E 3rd, Oncale M, Safah H, Schmieg J. Therapy-related T/Myeloid mixed phenotype acute leukemia in a patient treated with chemotherapy for cutaneous diffuse large B cell lymphoma. J La State Med Soc. 2016; 168:16–20. PMID: 26986862.
4. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114:937–951. PMID: 19357394.
5. Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995; 9:1783–1786. PMID: 7564526.
6. Cho YU, Chi HS, Park CJ, Jang S, Seo EJ. Rapid detection of prognostically significant fusion transcripts in acute leukemia using simplified multiplex reverse transcription polymerase chain reaction. J Korean Med Sci. 2012; 27:1155–1161. PMID: 23091311.
7. Matutes E, Pickl WF, Van’t Veer M, Morilla R, Swansbury J, Strobl H, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011; 117:3163–3171. PMID: 21228332.
8. Carbonell F, Swansbury J, Min T, Matutes E, Farahat N, Buccheri V, et al. Cytogenetic findings in acute biphenotypic leukaemia. Leukemia. 1996; 10:1283–1287. PMID: 8709632.
9. Demiriz IŞ, Tekgünduz E, Bozdağ SC, Altuntaş F. Chronic myeloid leukemia as a secondary malignancy following treatment of diffuse large B-cell lymphoma. Turk J Haematol. 2014; 31:92–84. PMID: 24764737.
10. Inukai T, Sugita K, Mitsui K, Iijima K, Goi K, Tezuka T, et al. Participation of granulocyte colony-stimulating factor in the growth regulation of leukemia cells from Philadelphia chromosome-positive acute leukemia and blast crisis of chronic myeloid leukemia. Leukemia. 2000; 14:1386–1395. PMID: 10942233.
Fig. 1
Bone marrow aspirate smear showing leukemic blasts. (A) Increased blasts with medium cell size, oval to round shape, vesicular nuclei, fine chromatin patterns, and basophilic cytoplasm (×1,000, Wright-Giemsa stain), (B) More than 3% of blasts were positive for myeloperoxidase (×1,000, myeloperoxidase stain).
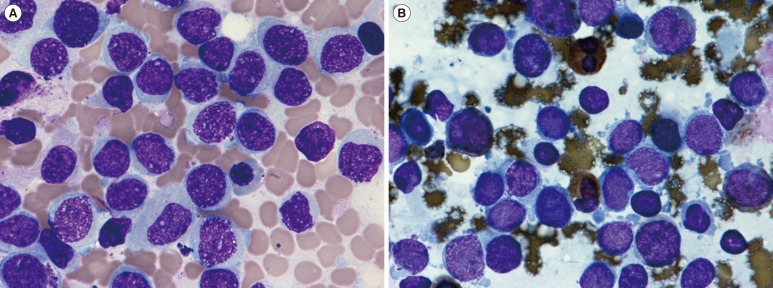
Fig. 2
Flow cytometry analysis of the bone marrow cells (Cytomics FC500 flow cytometer, Beckman Coulter, Fullerton, CA, USA). By CD45/SS linear gating, a relatively homogenous population of blasts consist 38.46% of all bone marrow cells. These cells were positive for both T-lymphoid (cytoplasmic CD3, CD5, and CD7) and myeloid differentiation (cytoplasmic MPO, CD13, and CD33) markers.
Abbreviations: FITC, fluorescein isothiocyanate; MPO, myeloperoxidase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download