Dear Editor,
Inflammatory bowel diseases (IBDs) such as Crohn's disease (CD) and ulcerative colitis (UC) are chronic relapsing inflammatory disorders of the intestine that are characterized by abdominal pain and diarrhea [12]. The incidence of IBD is increasing globally and has also increased in Korea recently [23]. Although the etiology is unknown, the relationship between the colonic microbiota and IBD has been indicated as a possible contributor. Fusobacterium varium and F. nucleatum have emerged as compelling candidates responsible for IBD exacerbation [45]. The colonization by these strains is possibly a useful biomarker for diagnosing gastrointestinal diseases [5]. We aimed to determine the prevalence of Fusobacterium spp. from colonic biopsies of IBD patients in Korea.
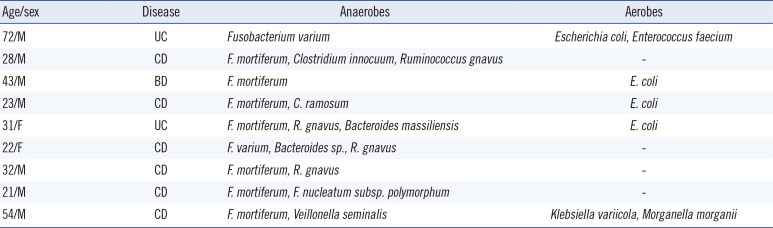
All 54 patients fulfilling the diagnostic criteria for IBD including 26 of CD, 25 of UC, or 3 of Behcet's disease (BD), at Hanyang University Guri Hospital between June 2014 and June 2015 were included in this study. Among them, 41 (76%) were male. The patient ages ranged from 14 to 75 yr (median: 31 yr). The Institutional Review Board of Hanyang University Guri Hospital approved this study. Biopsy material was obtained from patients undergoing colonoscopy for assessment of IBD status or for confirmation of gastrointestinal disease. Tissues were put into pre-reduced phosphate-buffered saline and immediately transported to the laboratory for bacterial culture. The specimens were ground, inoculated on crystal violet erythromycin (CVE) agar supplemented with tryptophan, and incubated at 37℃ for 48 hr in an anaerobic jar [6]. All isolates showing a different colony morphology were identified by 16S rRNA gene sequencing by using PCR predicted to yield a product of 1,300 bp [7]. The PCR conditions were as follows: 95℃ for 5 min; 30 cycles at 95℃ for 30 sec, 55℃ for 30 sec, and 72℃ for 1 min; and a final 10-min step at 72℃. The sequencing of the amplification products were performed in a commercial facility (Macrogen, Seoul, Korea). The sequencing analysis was completed using the GenBank and EzTaxon databases. Some strains were identified using the MicroScan WalkAway automated system (Dade Behring, West Sacramento, CA, USA) and the Vitek MS system (bioMérieux, Marcy l'Etoile, France). Ten Fusobacterium isolates were recovered from nine of the 54 patients as follows: six F. mortiferum, two F. varium, and one F. nucleatum and F. mortiferum (Table 1).
Clinically, the most important Fusobacterium species are F. nucleatum and F. necrophorum, but F. ulcerans and the F. mortiferum–F. varium group have occasionally been isolated from human. Ohkusa et al. [4] suggested that F. varium might be one of the elusive pathogenic factors in UC. In addition, Strauss et al. [5] reported that an F. nucleatum strain isolated from IBD tissues was more invasive than strains isolated from normal tissues. A correlation between UC and colonization with F. varium has also been noted in a cohort of Japanese patients [4]. F. nucleatum has been linked to colorectal cancer as well as IBD, which has been recognized as a risk factor of colorectal cancer [8]. Fusobacterium spp. were isolated from 63.6% and 53.1% of IBD patients in Canada and Japan, where F. nucleatum was more prevalent in the Canadian patients (43%) than in the Japanese patients (4%) [59]. The prevalence of Fusobacterium in our study cohort was 17%, which was lower than that in Canada and Japan.
There were limitations of our study that could have affected the low recovery rate. First, whereas we allowed only two days of incubation time for anaerobic culture, Strauss et al. [5] allowed five days. Hence, it was possible that some Fusobacterium spp. were lost owing to early termination of the incubation. Furthermore, with a longer incubation time, the anaerobic chamber may be more optimal than the anaerobic jar that we used. Second, for the specific recovery of Fusobacterium spp. from clinical specimens, the josamycin, vancomycin, and norfloxacin in JVN agar are better than the CVE agar that we used [10]. Further studies are necessary because of our small sample size and the absence of strains isolated from healthy controls. Although it is not possible to draw any strong conclusions from this small data subset, it is likely that there are differences of isolated species according to the country of origin, supporting the notion that no single bacterial species is a biomarker of IBD.
In conclusion, this study is the first attempt to assess the prevalence of Fusobacterium spp. in colonic tissues of IBD patients in Korea, revealing that only 17% of the patients carried species of this genus.
Acknowledgments
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120176).
References
1. Maloy KJ. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011; 474:298–306. PMID: 21677746.

2. Lee JW, Im JP, Cheon JH, Kim YS, Kim JS, Han DS. Inflammatory bowel disease cohort studies in Korea: Present and Future. Intest Res. 2015; 13:213–218. PMID: 26130995.

3. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54.e42. quiz e30PMID: 22001864.

4. Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003; 52:79–83. PMID: 12477765.
5. Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, Devinney R, et al. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011; 17:1971–1978. PMID: 21830275.

6. Walker CB, Ratliff D, Muller D, Mandell R, Socransky SS. Medium for selective isolation of Fusobacterium nucleatum from human periodontal pockets. J Clin Microbiol. 1979; 10:844–849. PMID: 521483.
7. Clinical and Laboratory Standards Institute. 2008. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing. Approved standard MM18-A. 1st ed. Wayne, PA: Clinical and Laboratory Standards Institute.
8. Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012; 22:299–306. PMID: 22009989.
9. Tahara T, Shibata T, Kawamura T, Okubo M, Ichikawa Y, Sumi K, et al. Fusobacterium detected in colonic biopsy and clinicopathological features of ulcerative colitis in Japan. Dig Dis Sci. 2015; 60:205–210. PMID: 25102986.
10. Brazier JS, Citron DM, Goldstein EJ. A selective medium for Fusobacterium spp. J Appl Bacteriol. 1991; 71:343–346. PMID: 1960109.
Table 1
Fusobacterium species and other bacteria isolated from nine patients, identified by using 16S rRNA gene sequencing




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download