XYY syndrome is a rare sex chromosome abnormality that occurs in 1 of 1,000 male births, and shows a normal phenotype with the exception of increased stature [1]. However, other clinical characteristics such as psychological dysfunction [2] and urinary tract and hepatobiliary system abnormalities have been reported with XYY syndrome [3]. Here, we describe the first case of XYY syndrome with the occurrence of a cervical immature teratoma and subsequent acute promyelocytic leukemia (APL).
The patient was born as a full-term baby and had an irregular-shaped mass measuring 10 cm, on the left side of his neck. Magnetic resonance imaging showed a well-defined, enhanced mass, measuring 5.6×4.9×4.5 cm in size (Fig. 1A). Alpha-fetoprotein (AFP) in the blood was markedly above the normal ranges (30,066.0 IU/mL). Conventional cytogenetics of peripheral blood revealed a 47,XYY karyotype, indicating an extra Y chromosome. Pathological diagnosis of the excised mass confirmed an immature teratoma, grade 3 (Fig. 1B and C). The patient was treated with three cycles of cisplatin (10 mg/m2 for 5 days) and etoposide (50 mg/m2 for 5 days) after surgical resection. The serum AFP level after the first cycle of chemotherapy was 289.3 IU/mL, and the level was normalized after four months of age.
Twenty-eight months after completion of chemotherapy, the patient showed multiple bruises on his extremities and bicytopenia (white blood cells: 6.3×109/L, hemoglobin: 8.4 g/dL, and platelets: 18×109/L). Bone marrow (BM) aspiration showed that 43% of the promyelocytes were abnormal (Fig. 1D and E). Flow cytometric analysis of BM cells revealed a clonal myeloid cell population that was positive for CD13 (60.4%), CD33 (58.7%), and MPO (96.4%), and negative for CD34 (4.1%) and HLA-DR (1.9%).
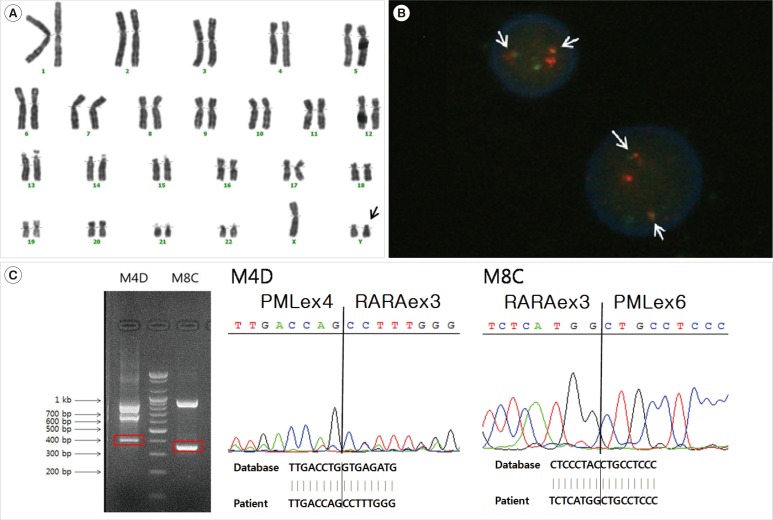
Conventional cytogenetics of BM cells revealed a 47,XYY karyotype without abnormalities in chromosomes 15 and 17 (Fig. 2A). However, a PML/RARA rearrangement was detected by FISH (Fig. 2B), multiplex reverse-transcription PCR (RT-PCR) (HemaVision kit; DNA-Diagnostic, Risskov, Denmark), and quantitative real-time PCR (qRT-PCR). We confirmed PML/RARA rearrangement by direct sequencing (Fig. 2C).
The patient was treated with ATRA, and after the first induction chemotherapy, morphological remission was achieved with a decrease in PML-RARA/ABL, as determined by qRT-PCR. After two cycles of consolidation chemotherapy, the patient began maintenance chemotherapy.
According to recent reports, the incidence of cancer in the XYY syndrome population is not significantly different from that in the general population; however, mortality from cancer is significantly higher [14].
Hematologic malignancies are the most commonly reported neoplasms associated with XYY syndrome, and other neoplasms are reported rarely [5]. In this case, APL occurred 28 months after chemotherapy for the teratoma. Therapy-related AML occurs with a latency period of one to five years after treatment with topoisomerase II inhibitors and other chemotherapeutics. It is often associated with balanced chromosomal translocations, and characteristics and outcomes are not very different from those of de novo AML [9]. Etoposide-related AML occurs mostly in patients treated with a high cumulative dose (>2,000 mg/m2) [7]. In this patient, because of etoposide use, the 28-month interval, and the PML-RARA rearrangement, therapy-related APL was suspected. However, the cumulative dose of etoposide was much lower than 2,000 mg/m2. Therefore, we also considered other possibilities such as malignant transformation of the teratoma to leukemia [8] or the genetic vulnerability of hematopoietic tissue itself to chemotherapy in XYY syndrome cases. Leukemia associated with a teratoma is reported to occur simultaneously or within a short interval of about six months (range, 1-22 months) [9]. It is presumed that hematopoietic microfoci within the primary teratoma tissue might be the origin of the leukemia; this hypothesis is supported by identical clonal aberrations in both teratoma and leukemia [9]. A more likely explanation for pathogenesis is an association with the effects of XYY syndrome itself. First, dysfunction or malformation of the kidneys and liver are common abnormalities in patients with XYY syndrome, making them more susceptible to cytotoxic complications of chemotherapy [10]. Second, constitutional aberrations might result in a high susceptibility to potentially leukemia-inducing agents. Considering these possibilities, careful administration of chemotherapy dose and attentive follow-up examinations are recommended when treating neonates with XYY syndrome.
In summary, although further evaluations in large-scale epidemiological studies will be required to demonstrate the association between XYY syndrome, teratoma, and subsequent leukemia, our case showed a new phenotype, the presentation of teratoma and APL, in the spectrum of disease in pediatric XYY syndrome.
Acknowledgments
This study was supported by grants from the National Research Foundation of Korea (NRF) (No. 2011-0015304), and the Leading Foreign Research Institute Recruitment Program (No. 2011-0030034) through the NRF funded by the Ministry of Education, Science and Technology (MEST), and a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (No. 2013-1320070). We thank Seung Yeob Lee who provided advice during the drafting of the manuscript, and Sung-Du Jeong and Jae-Sung Lee who provided technical support for molecular genetic studies.
References
1. Stochholm K, Juul S, Gravholt CH. Diagnosis and mortality in 47,XYY persons: a registry study. Orphanet J Rare Dis. 2010; 5:15. PMID: 20509956.

2. Jacobs PA, Brunton M, Melville MM, Brittain RP, McClemont WF. Aggressive behavior, mental sub-normality and the XYY male. Nature. 1965; 208:1351–1352. PMID: 5870205.
3. Côté GB, Tsomi K, Papadakou-Lagoyanni S, Petmezaki S. Oligohydramnios syndrome and XYY karyotype. Ann Genet. 1978; 21:226–228. PMID: 314260.
4. Higgins CD, Swerdlow AJ, Schoemaker MJ, Wright AF, Jacobs PA. UK Clinical Cytogenetics Group. Mortality and cancer incidence in males with Y polysomy in Britain: a cohort study. Hum Genet. 2007; 121:691–696. PMID: 17457613.

5. Limacher JM, Girard-Lemaire F, Jeandidier E, Chenard-Neu MP, Kassem M, Flori E, et al. Gastrointestinal stromal tumor in an XYY/XY male. Cancer Genet Cytogenet. 2002; 133:152–155. PMID: 11943343.

6. Swerdlow SH, Campo E, editors. WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer;2008. p. 127–129.
7. Schneider DT, Hilgenfeld E, Schwabe D, Behnisch W, Zoubek A, Wessalowski R, et al. Acute myelogenous leukemia after treatment for malignant germ cell tumors in children. J Clin Oncol. 1999; 17:3226–3233. PMID: 10506623.

8. Harms D, Zahn S, Göbel U, Schneider DT. Pathology and molecular biology of teratomas in childhood and adolescence. Klin Padiatr. 2006; 218:296–302. PMID: 17080330.

9. Nichols CR, Roth BJ, Heerema N, Griep J, Tricot G. Hematologic neoplasia associated with primary mediastinal germ-cell tumors. N Engl J Med. 1990; 322:1425–1429. PMID: 2158625.

10. Shibata S, Kami M, Kishi Y, Hamaki T, Ueyama JI, Miyakoshi S, et al. Severe regimen-related toxicity occurring in a patient with XYY syndrome receiving allogeneic peripheral blood stem cell transplantation. Ann Hematol. 2002; 81:407–409. PMID: 12185516.

Fig. 1
Radiographical and morphological findings of teratoma and bone marrow study. (A) A coronal magnetic resonance image (MRI) at birth showed a unilateral, large, well-defined, and enhanced mass. (B) A well-circumscribed and irregular-shaped mass was extirpated from left side of the neck (7 cm×5 cm). (C) The tissue biopsy showed an immature teratoma with primitive neuroepithelial rosettes lined by basophilic cells (hematoxylin and eosin stain, ×100). (D) Bone marrow findings at diagnosis showed depleted trilineage cellular component, proliferation of abnormal promyelocytes with large purple granules and irregular-shaped (kidney-shaped or bilobed) nuclei (Wright-Giemsa stain, ×400). (E) High magnification images from (D) disclosed abnormal promyelocytes (Wright-Giemsa stain, ×1,000).
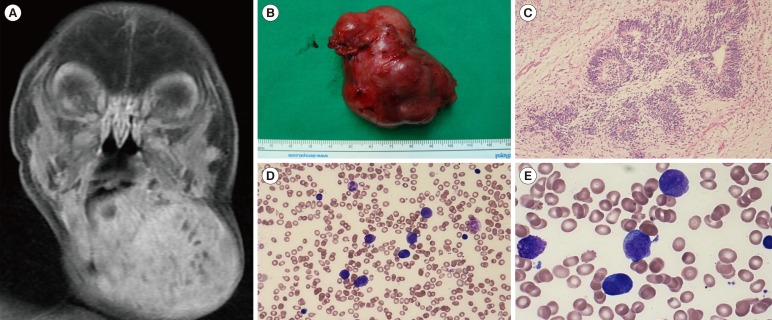
Fig. 2
Cytogenetic and molecular studies at diagnosis of acute promyelocytic leukemia. (A) Giemsa-banded karyotype of bone marrow showing 47,XYY chromosomal arrangement. Additional Y (in arrow) and intact chromosomes 15 and 17 are observed. (B) Dual-color FISH with PML-RARA probes demonstrates the presence of PML-RARA fusion (in arrows). (C) Direct sequencing for the confirmation of PML-RARA rearrangement shows that the 396-bp band of M4D contains a fusion transcript of PML exon 4-RARA exon 3 and that the 353-bp band of M8C contains a fusion transcript of PML exon 6-RARA exon 3. The fusion transcript of PML exon 4-RARA exon 3 is a result of alternative splicing of the PML exon 6-RARA exon 3 fusion gene.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download