Abstract
Thyroid disorders are common, affecting more than 10% of people in the US, and laboratory tests are integral in the management of these conditions. The repertoire of thyroid tests includes blood tests for thyroid-stimulating hormone (TSH), free thyroxine, free triiodothyronine, thyroglobulin (Tg), thyroglobulin antibodies (Tg-Ab), thyroid peroxidase antibodies (TPO-Ab), TSH receptor antibodies (TRAb), and calcitonin. TSH and free thyroid hormone tests are frequently used to assess the functional status of the thyroid. TPO-Ab and TRAb tests are used to diagnose Hashimoto's thyroiditis and Graves' disease, respectively. Tg and calcitonin are important tumor markers used in the management of differentiated thyroid carcinoma and medullary thyroid carcinoma (MTC), respectively. Procalcitonin may replace calcitonin as a biomarker for MTC. Apart from understanding normal thyroid physiology, it is important to be familiar with the possible pitfalls and caveats in the use of these tests so that they can be interpreted properly and accurately. When results are discordant, clinicians and laboratorians should be mindful of possible assay interferences and/or the effects of concurrent medications. In addition, thyroid function may appear abnormal in the absence of actual thyroid dysfunction during pregnancy and in critical illness. Hence, it is important to consider the clinical context when interpreting results. This review aims to describe the above-mentioned blood tests used in the diagnosis and management of thyroid disorders, as well as the pitfalls in their interpretation. With due knowledge and care, clinicians and laboratorians will be able to fully appreciate the clinical utility of these important laboratory tests.
Go to : 
Thyroid conditions are among the most common endocrine disorders. Laboratory tests are integral in the diagnosis and management of most of these conditions. Sometimes, thyroid imaging, such as thyroid ultrasound or radionuclide scans, may be needed for disease management. In addition, thyroid autoantibodies are frequently tested to diagnose autoimmune thyroid diseases, such as Hashimoto's thyroiditis and Graves' disease. Thyroglobulin (Tg) and calcitonin are used as tumor markers in differentiated thyroid carcinoma (DTC) and medullary thyroid carcinoma (MTC), respectively. Thyroid function tests (TFTs) are the most commonly ordered endocrine tests in both inpatient and outpatient settings; at our institution (Changi General Hospital, Singapore), TFTs constitute more than 60% of endocrine tests. The annual number of thyroid-stimulating hormone (TSH) tests ordered in the US according to a 2013 report was 59 million, while that of free thyroxine (FT4) tests was 18 million [1]. The annual cost for these two tests alone in the US is estimated at $1.6 billion, and there is wide practice variation in the ordering of tests for thyroid dysfunction [2]. In general, it is not difficult to interpret these laboratory tests. However, when the results are discordant or incongruous with the clinical picture, their interpretation can be challenging. This review covers the various laboratory tests used in the diagnosis and management of thyroid conditions, illustrates the pitfalls in their interpretation, high-lights their utility in clinical practice, and provides guidance for rational test ordering.
Go to : 
Thyroid hormone synthesis is tightly regulated by the hypothalamus-pituitary-thyroid axis. In healthy subjects, thyrotropin-releasing hormone (TRH) from the hypothalamus stimulates the secretion of TSH from the anterior pituitary gland. TSH in turn stimulates the production of thyroxine (T4) and triiodothyronine (T3), which account for 85–90% and 10–15% of thyroid hormones, respectively, in the thyroid gland [3]. T3 is the bioactive thyroid hormone and is largely derived from peripheral conversion of T4 under the action of deiodinases. More than 99% of T4 and T3 molecules are tightly bound to the carrier proteins, thyroid binding globulin (TBG), transthyretin, and albumin, and only a very small percentage circulates as free hormones. These free hormones act on target tissues by binding onto thyroid receptors in the nuclei of target cells. In addition, they provide negative feedback to both the hypothalamus and the pituitary gland, closing the tightly regulated homeostatic thyroid hormone synthesis loop. The TSH-free thyroid hormone relationship is inversely log-linear [3]. TSH secretion is very sensitive to minor fluctuations in thyroid hormone levels, and abnormal TSH levels are associated with early thyroid dysfunction, before actual thyroid hormone abnormalities occur. The TSH-FT4 relationship is genetically determined [4] and is influenced by age, smoking, and thyroid antibody status [5]. Despite some reservations [56], the TSH-FT4 relationship is largely inversely log-linear, as indicated by a recent study of 13,379 subjects [7]. In fact, this relationship is even stronger when FT4 is measured by tandem mass spectrometry instead of immunoassay [8].
Go to : 
TSH, a dimeric glycoprotein, comprises an alpha chain (92 amino acids) in common with human chorionic gonadotrophin (hCG), follicle-stimulating hormone (FSH), and luteinizing hormone (LH), and a unique beta subunit (118 amino acids). Improvements in TSH technology have largely eliminated any alpha-subunit cross-reactivity. Its secretion follows a circadian pattern, with the nadir in the late afternoon and peak between midnight and 4 am [910]. Different analytical platforms quote different TSH reference ranges. According to the US National Health and Nutrition Examination Survey III, in a large (N=13,444) disease-free and thyroid peroxidase antibody (TPO-Ab) negative population, the upper reference limit for TSH was 4.5 mIU/L [11]. A Singapore-based study (N=872) reported a TSH reference range of 0.4–3.9 mIU/L [12] on the Vitros ECi platform (Ortho Clinical Diagnostics, Rochester, New York, USA), which is fairly similar to the range of 0.4–4.0 mIU/L that is widely used in Europe [13].
TSH tests are recommended as first-line screening tests for thyroid dysfunction [1415]. Highly sensitive third-generation immunometric assays (sandwich or non-competitive assays), which are capable of detecting TSH levels <0.01 mIU/L, have been widely used since the late 1980s [16]. In the Singapore study cited above, the Vitros assay had a sensitivity of 0.005 mIU/L [12]. However, caution must be exercised in the sole use of TSH tests for subclinical thyroid dysfunction and for secondary hypothyroidism in hospitalized patients and during the initial phases of medical therapy for hyper- and hypothyroidism [17]. In these situations, it is pertinent to test free thyroid hormones as well. Otherwise, subclinical thyroid dysfunction may be wrongly diagnosed as overt thyroid dysfunction, secondary hypothyroidism, and non-thyroidal illness as primary hyperthyroidism or euthyroidism (depending on TSH levels). Moreover, as TSH responses tend to lag behind improvement in free thyroid hormone levels, it is essential to monitor the latter in the initial treatment of hyper- and hypothyroidism to adjust the medication doses appropriately. It is also important to note that TSH levels may increase with age because of relatively higher levels of biologically inactive isoforms of TSH [18]. In addition, elevated TSH due to thyroid gland and pituitary dysfunction among the elderly (>80 years) in Baltimore, USA, has been reported [19]. In Australian subjects (N=908) free of thyroid disease and thyroid antibodies, tested on Immulite 2000 (Siemens Healthcare Diagnostic Products, Deerfield, IL, USA), aging was associated with increased TSH without concomitant alteration in FT4 over a 13-year period [20]; it suggested that the increase in TSH had arisen from reduced TSH bioactivity or age-related alteration in the TSH-FT4 set point. Notably, the largest increases in TSH (of 0.4–0.6 mIU/L) in that study were observed in people with the lowest baseline TSH (of <0.9 mIU/L).
In the past, most tests of thyroid hormone production assessed total thyroid hormone levels [21]. However, as thyroid hormones are mostly bound to carrier proteins (mainly TBG), conditions that affect TBG levels (e.g., pregnancy and acute illness) will result in abnormal total thyroid hormone levels in the absence of actual thyroid dysfunction. In addition, certain medications cause alterations in TBG levels (e.g., increased levels with estrogen, tamoxifen, and opiates, and decreased levels with androgens and glucocorticoids). Thyroid hormones are not significantly affected by changes in the other thyroid hormone binding proteins, transthyretin, and albumin. However, total T4 is increased (with variable elevations in free T4) in familial dysalbuminemic hyperthyroxinemia (FDH) [2223]. FDH is a rare autosomal dominant condition associated with an albumin variant with increased affinity for T4, but not T3, in clinically euthyroid subjects. Testing for free thyroid hormones as the biologically active hormones has superseded total thyroid hormone testing. In the US, FT4 tests account for 90% of T4 testing [2]. As FT3 and FT4 circulate in picomolar levels, while total thyroid hormones (TT3 and TT4) are present in nanomolar levels, free thyroid hormones are more difficult to measure accurately and precisely [1].
Previously, free thyroid hormone levels were estimated indirectly, such as resin uptake test, FT4 index, and T4/TBG ratio. To measure the minute quantities of free hormones directly, the bound thyroid hormones have to be removed. While direct measurements of FT3 and FT4 are more accurate when preceded by equilibrium dialysis and ultrafiltration, the technical complexity and high cost of such methods have relegated them to research use only. Most laboratories use indirect measurements by competitive immunoassays. Free thyroid hormones are extracted from serum with specific high-affinity antibodies. Unbound serum constituents are removed, and the antibody-bound thyroid hormones are incubated with a labeled thyroid hormone probe. The unoccupied antibody-binding sites are inversely proportional to the free thyroid hormone level [12223]. This two-step FT4 assay format is preferred over one-step assays as non-specific binding is reduced and as T4 analogues are not employed. This is exemplified in a recent case report of T4 autoantibodies affecting a one-step fT4 assay (Advia Centaur XP, Siemens Healthcare, Munich, Germany) but not the two-step fT4 assay (Architect i2000, Abbott Diagnostics, Santa Clara, CA, USA) [24]. As FT4 circulates in the bloodstream at levels two to three times higher than those of FT3, the precision of the FT4 assay is better than that of FT3 assays, as expected [12].
Tg is a 660-kDa homodimeric glycoprotein that is produced by thyroid follicular cells. It is an important tumor marker for DTC following total thyroidectomy and radioiodine remnant ablation. Most clinical laboratories use immunometric assays to measure serum Tg. These assays should be calibrated according to the Certified Reference Materials and Methods-457 international standard to minimize variations [25]. An exemplary reference study [26] quotes a serum Tg of 29 ng/mL for men and 38 ng/mL for women on the Beckmann-Coulter DXI 800 immunoassay system (Beckmann-Coulter SA, Nyon, Switzerland). All subjects (M=209, F=229) had no personal or familial history of thyroid disease, normal TSH (0.5–2.0 mIU/L), negative thyroid antibodies, and normal thyroid ultrasound. One of the caveats of using immunometric Tg assays is potential interference by thyroglobulin antibodies (Tg-Ab), which are present in up to 25% of patients with DTC [27]. Elevated Tg-Ab can lead to falsely low levels of serum Tg. Hence, serum Tg should always be measured together with Tg-Ab during follow-up for DTC [28]. Greater emphasis should be placed on imaging (thyroid ultrasound, I-131 whole body scan), or even fluorodeoxyglucose-positron emission tomography-computerized tomography (FDG-PET CT) scan in patients with elevated Tg-Ab levels. Even though there is less interference from Tg-Ab when radioimmunoassays are used, these assays are not widely employed because of their lower sensitivity and longer assay times [13].
Serum Tg-Ab is a marker of thyroid autoimmunity. Since serum Tg-Ab is elevated in 10% of the general population (especially in women), it is not as sensitive or specific as a thyroid biomarker compared with thyroid peroxidase antibodies (TPO-Ab) or TSH receptor antibodies (TRAb) [25]. In the absence of TPO-Ab, Tg-Ab is not significantly associated with thyroid disease [11]. The main clinical utility of the Tg-Ab test is to ensure the reliability of the serum Tg test in the follow-up of patients with DTC. For patients with elevated Tg-Ab (which renders serum Tg unreliable as a tumor marker), Tg-Ab itself can serve as a surrogate tumor marker for DTC [25272829].
TPO-Ab is found in 5–20% of the general population and is nearly always elevated in patients with Hashimoto's thyroiditis [11]. Apart from aiding in the diagnosis of Hashimoto's thyroiditis, TPO-Ab may play a role in the management of subclinical hypothyroidism. In a 20-year follow-up study in the UK, progression to overt hypothyroidism occurred at 4.3% per years in patients with elevated TPO-Ab (measured as antimicrosomal antibodies), compared with 2.6% per years in those who were TPO-Ab-negative [30]. This has also been found in patients from the USA [31]. In an Australian study using contemporary TPO-Ab assays [32], women with elevated TPO-Ab (above 29 kIU/L) (using Immulite 2000, Siemens Healthcare Diagnostic Products) and TSH between 2.5 and 4.0 mIU/L progressed to subclinical and overt hypothyroidism after 13 years. Hence, patients with subclinical hypothyroidism and elevated TPO-Ab may need close monitoring or even be started on thyroid hormone replacement. In addition, the American Thyroid Association recommends that pregnant euthyroid women with elevated TPO-Ab should have their TSH checked monthly to decide on treatment during pregnancy [33].
TRAb binds to the TSH receptors. There are three types of TRAb: stimulating, blocking, or neutral, of which thyroid-stimulating antibodies are the most common [34]. TRAb can be measured using two types of assays: competitive TSH-binding inhibition (TBI) or thyroid-stimulating immunoglobulin (TSI) assays [35]. The former measures TRAb in serum samples based on its ability to inhibit the binding of TSH receptors with known TSH receptor ligands. This assay cannot differentiate stimulating from the other two types of TRAb [34]. The TSI assay employs cAMP production in cells incubated with patient serum. This assay can identify only stimulating TRAb [35]. As TRAb is not present in the general population, it is specific for the diagnosis of Graves' disease. TRAb has been used in the differential diagnosis of hyperthyroidism, prediction of remission after treatment of Graves' hyperthyroidism, prediction of fetal/neonatal thyrotoxicosis, and assessment of ophthalmopathy [35]. A fully automated TBI assay has been available for the last 10 years [36]. Recently, an automated assay for TSI has been introduced [3738].
Calcitonin is a 32-amino-acid polypeptide hormone produced by the parafollicular C cells of the thyroid gland. It is derived from proteolytic cleavage of its precursor peptides pre-procalcitonin and procalcitonin (PCT, 116 amino acids). In the clinical setting, it is mainly utilized as a tumor marker for MTC. While advances in calcitonin assays have largely eliminated cross-reactivity with procalcitonin, calcitonin can be elevated in several conditions [394041], such as C-cell hyperplasia, autoimmune thyroiditis, hypercalcemia, chronic renal failure, bacterial infections, pregnancy and lactation, and malignancy (e.g., lung, breast, pancreas, leukemia, and systemic mastocytosis), and by certain medications (e.g., proton pump inhibitors and glucagon-like peptide-1 agonists). Further, calcitonin release can also be stimulated by food [39]. Samples for calcitonin testing need to be drawn in the morning after an overnight fast, transported to the laboratory on ice, processed rapidly, and frozen before analysis. Given the rarity of MTC, requests for calcitonin assays are naturally low, and laboratories often send samples out to referral centers for analysis. Moreover, there are several technical challenges in calcitonin assays, including sample stability, biphasic half-life (<30 minutes at physiological levels and up to 30 hours at high levels), and presence of calcitonin isoforms and fragments [42]. The calcitonin precursor PCT is free from such problems and circulates at a 100-fold greater levels, rendering its measurement more precise. PCT and calcitonin are strongly correlated in MTC patients, and they have similar diagnostic performance [4243]. A recent systematic review affirmed that PCT plays an important role in the management of MTC [44]. Elevated PCT in sepsis and inflammatory conditions will certainly confound its use in MTC.
Go to : 
A concrete understanding of thyroid physiology and the various thyroid tests suffices for the proper and accurate interpretation of their results in most clinical situations. However, TFT results should be interpreted cautiously, considering laboratory assay interferences, concurrent medications, pregnancy, non-thyroidal illness, and elderly patients.
Many factors can interfere with laboratory tests, and TFTs are no exception. The presence of human anti-animal antibodies in patient serum may interfere with TSH measurement [45]. If the antibodies block TSH binding to capture or detection antibodies in the assays, negative interference will occur, leading to falsely low TSH levels. By contrast, if the antibodies cross-link with capture and detection antibodies, positive interference will occur, resulting in falsely elevated TSH levels. Heterophile antibodies, such as rheumatoid factor, may lead to similar assay interferences. Interfering auto-antibodies to T4 have also been reported to falsely elevate FT4 levels [24]. Most manufacturers now incorporate various blocking agents in their assays to avert this problem.
The use of high-dose biotin (100–300 mg/day) for multiple sclerosis and inherited metabolic disorders has attracted the attention of laboratorians and clinicians, as it can cause inexplicable thyroid test results [4647]. In addition, biotin is touted for healthy nails and hair, and it may be present in supplements for this purpose in doses of up to 10 mg per tablet.
Streptavidin and biotin are commonly employed in immunoassay platforms to capture antigens (e.g., TSH and FT4) or antibodies (e.g., TRAb) onto a solid phase. In competitive assays (e.g., FT4 assay), biotinylated T4 competes with serum FT4 for binding sites on a T4-specific antibody labeled with a signaling molecule, for example, ruthenium. The biotinylated T4-ruthenium-antibody complex is trapped on a solid phase (streptavidin microparticles). The microparticle-antibody-antigen complex is magnetically captured onto an electrode. Following voltage application to the electrode, the chemiluminescent signal generated is inversely proportional to the FT4 levels in the sample. High biotin levels in the serum sample will inhibit the formation of the solid phase complex, resulting in a low signal. This will produce a falsely high FT4 result [48]. In sandwich assays (e.g., for TSH), the serum sample is incubated with a biotinylated TSH-Ab. A second ruthenium-labeled TSH-specific antibody is added. The resulting biotinylated TSH-Ab-ruthenium-Ab complex is trapped on a streptavidin microparticle solid phase and captured onto an electrode. The chemiluminescent signal generated is proportional to the sample TSH levels. High amounts of biotin in the sample will prevent the formation of the solid phase complex, yielding a low or absent signal. This will produce a falsely low TSH result. The combination of low TSH and high FT4 gives a false impression of hyperthyroid results on the ruthenium chemiluminescence assay system. Moreover, the confounding effect of biotin will be even stronger when accompanied by a falsely high TRAb result, which is a competitive assay format.
Fortunately, most multivitamins for adults in the market contain only a small amount of biotin (<1–3 mg per tablet) and will not cause assay interference. However, patients with rare inborn metabolic errors (e.g., biotin-thiamine-responsive basal ganglia disease or biotin cycle defects) require megadoses of 10–15 mg/kg of biotin daily. In these patients, possible interference can be confirmed by asking the patient directly about their medical history and consumption of supplements or retesting on biotin-free automated immunoassays. Another approach to overcoming such interference is biotin neutralization by pre-treatment of the patient sample with streptavidin [4749].
Medications can affect TFTs, and appreciation of their effects is vital. Apart from medications that affect TBG levels mentioned above, numerous other commonly used medications can cause altered thyroid function in other ways [5051]. Dopamine agonists, glucocorticoids, somatostatin analogues, and metformin have been associated with a decrease in pituitary TSH secretion [5253]. Frusemide (especially at high intravenous doses), salicylates, phenytoin, and heparin are known to compete for thyroid hormone-binding sites on carrier proteins [45]. Lithium and tyrosine kinase inhibitors can lead to primary hypothyroidism [54]. It is worth noting that discordant TFT results may be seen in patients treated for established thyroid disorders (especially in the early phases of treatment or after changes in medication dosages) or given thyroid hormones. Such information is often not conveyed to the laboratory. This is amply illustrated in a recent case report of a 31-year-old female, seen at a hospital outpatient clinic with no other details, who had TSH <0.01 mIU/L (0.35–5.5 mIU/L) and FT4 <1.3 pmol/L (11.5–22.7 mIU/L) [55]. Repeat testing on the same analyzer and a different immunoassay platform yielded similar results. Further investigations revealed that she had been prescribed T3 by her private doctor.
Amiodarone is a medication that merits special mention [56]. Due to its inhibition of type 1 deiodinases, initial use of amiodarone frequently results in transient, mild elevation of FT4, decrease in FT3, and elevation in TSH. Some patients living in iodine-sufficient areas may develop amiodarone-induced hypothyroidism (AIH), especially those with positive thyroid antibodies or underlying Hashimoto's thyroiditis. In iodine-deficient regions, amiodarone-induced thyrotoxicosis (AIT) is more common than AIH. There are two types of AIT, both of which require treatment [57]. Type 1 AIT results from the excess iodine in amiodarone, providing increased substrate for thyroid hormone synthesis (Jod-Basedow phenomenon) [57]. This phenomenon often occurs in patients with latent Graves' disease or pre-existing multinodular goiter. Type 2 AIT develops in apparently normal thyroid glands or small goiters. Amiodarone causes direct cytotoxicity on the thyroid follicular cells, with release of preformed thyroid hormones. The median time to onset of Type 1 AIT is 3.5 months, versus 30 months for Type 2 AIT [58]. However, the onset of AIH and AIT is not predictable, and thus, the value of regular TFT monitoring in patients on amiodarone is unclear [59].
Normal changes in thyroid physiology during pregnancy and the postpartum period can make TFT interpretation very challenging. Knowledge of these changes is needed for effective patient management. Owing to the structural homology between hCG and TSH, high levels of hCG during early pregnancy stimulate TSH receptors, resulting in 10–20% enlargement of the thyroid gland, 30% increase in thyroid hormone production, and a decrease in TSH levels [60]. The hyper-estrogenic state in pregnancy also increases hepatic TBG production, thus increasing the total thyroid hormone levels. Thyroid hormone levels plateau at the end of the first trimester. When hCG levels fall after the first trimester, free thyroid hormones decrease and TSH increases. TBG remains high until delivery. These dynamic changes require trimester-specific TFT reference ranges for the accurate assessment of thyroid status during pregnancy. If such reference ranges are not available, the American Thyroid Association (ATA) previously recommended using TSH ranges of 0.1–2.5, 0.2–3.0, and 0.3–3.0 mIU/L for the first, second, and third trimesters, respectively [61]. This recommendation has been revised in the latest ATA guidelines released in 2017 [33]. In the 2017 guidelines, the exact TSH ranges are not stipulated, but adjustments to the non-pregnant ranges are recommended instead, that is, reduction of lower and upper first-trimester TSH reference ranges by 0.4 mIU/L and 0.5 mIU/L, respectively. These TSH values should gradually return towards normal ranges in the subsequent trimesters. This has been borne out by a large Chinese study (N=1,409) that reported trimester-specific TSH ranges in thyroid-antibody-negative women of 0.2–3.8, 0.3–3.5, and 0.3–4.3 mIU/L for the first, second, and third trimesters, respectively, compared with 0.5–4.2 mIU/L in non-pregnant subjects [62].
In some studies, maternal hypothyroidism in early pregnancy was associated with poorer obstetric outcomes and fetal neurocognitive impairment [6364]. However, in a large study (N=2,411), first-trimester TSH in mothers with singleton pregnancies did not predict adverse events [63]. While maternal thyroid dysfunction can be readily diagnosed with trimester-specific ranges, and safe, effective treatment is available, the benefits of such interventions are not evident. Thus, universal thyroid screening in pregnancy remains controversial [64].
Women who have a history of autoimmune thyroid disease or other forms of autoimmunity may develop postpartum thyroiditis [33]. The incidence of postpartum thyroiditis has been estimated to be approximately 5.4% [65]. The course of postpartum thyroiditis follows a triphasic pattern and usually starts with transient hyperthyroidism, which occurs a few months after delivery. This is followed by transient hypothyroidism before eventual return to a euthyroid state. However, many patients do not follow this classical pattern. Some may have only isolated hyper- or hypothyroidism, and others may have permanent hypothyroidism. Most cases of postpartum thyroiditis do not require treatment. Hyperthyroid patients may be given β-blockers if symptomatic. Likewise, hypothyroid patients may be commenced on a course of levothyroxine if they are symptomatic, breastfeeding, or attempting to conceive [33]. Between 20% and 40% of patients with postpartum thyroiditis will develop permanent hypothyroidism [65]. These patients are likely to be older and to have higher thyroid antibody titers, higher TSH during the hypothyroid phase of the illness, multiparity, and hypo-echogenicity on thyroid ultrasound. Patients who fit this profile could be monitored more closely, as are those with symptoms of depression. In a 12-year follow-up study in Australia, postpartum thyroid dysfunction strongly predicted long-term hypothyroidism, and close follow-up was advised [66]. This same group identified low urinary iodide (<100 µg/L) as a risk factor [67].
Interpretation of thyroid function tests can be confounded by several factors in critically ill patients depending on the onset, severity, and duration of the critical illness [686970]. Understanding changes in thyroid hormones during illness will avert unnecessary testing and treatment. During critical illness, FT3 is the first to fall, typically within the first 24 hours. With time, FT4 also starts to fall, followed by a decrease in TSH. During recovery from the illness, TSH increases first and can often exceed the normal range. Normalization of free thyroid hormones will ensue. These changes in thyroid hormones in critical illness are believed to be brought about by several factors, such as reduced deiodinase activity, reduced thyroid hormone-binding protein concentrations, increased circulating pro-inflammatory cytokines, and concurrent use of certain medications, such as glucocorticoids. Whether these changes are a form of beneficial or maladaptive response remains unclear.
TSH tends to increase with age [7172]. This is due to alterations in thyroid metabolism and a gradual resetting of the hypothalamic-pituitary-thyroid axis. Diagnosing “real” hypothyroidism is challenging, as is distinguishing disease-specific symptoms from those of aging. For mildly elevated TSH levels (4–10 mIU/L) without elevated TPO-Ab, watchful waiting may be reasonable. Some laboratories have provided age-specific reference ranges [73]. However, their use has had minimal impact on thyroid status evaluation. In a very large reference population (N=148,938), there was only a marginal increase in TSH among patients classified by five-year age bands [74]. The 2.5th TSH percentile (0.5 mIU/L) was similar across age groups, while the 97.5th percentile increased from 3.75 mIU/L at age 40 to 5.0 mIU/L at age 90. In most age bands, these age-specific reference intervals reclassified only 0.1–1.9% of the subjects compared with the common cut-point of 4.0 mIU/L; a higher reclassification rate (2.1–4.7%) was found in subjects older than 85 years.
Go to : 
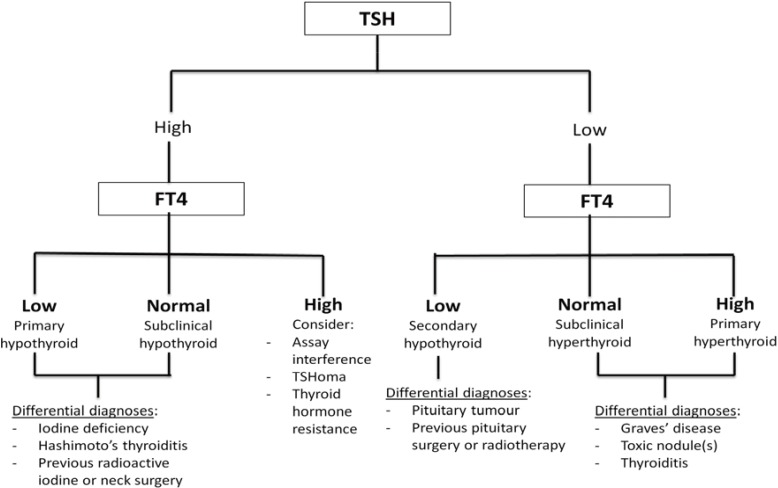
Symptoms of thyroid disease may be non-specific with minimal physical signs. The use of TFTs in patient evaluation is thus vital. While testing TSH alone is sufficient for general screening, both FT4 and TSH assays are needed for diagnosing subclinical thyroid dysfunction, central hypothyroidism, drug effects, and hospitalized patients, as well for accurate assessment of treatment effects. Close communication between the bedside and bench-side is crucial for successful interpretation of TFT results; an algorithm for TFT interpretation is presented in Fig. 1.
With the increased frequency of health screening and routine blood tests, more patients are diagnosed as having subclinical thyroid dysfunction. In subclinical hyperthyroidism, TSH level is low/suppressed, and FT4 level is within the normal range. FT3 level should be tested to rule out T3-toxicosis in these cases. Patients with subclinical hyperthyroidism should be further screened and considered for treatment, especially if they are elderly and/or at risk for atrial fibrillation and osteoporosis [71]. In subclinical hypothyroidism, TSH level is elevated and FT4 level is within the normal range. Levothyroxine should be commenced if the TSH level is markedly elevated (>10 mIU/L) or TPO-Ab is elevated. Treatment can also be considered in special situations, such as poorly controlled hypercholesterolemia and subfertility. It is important to note that even though the TSH test has been recommended as the first-line test for thyroid dysfunction, its sole utilization is insufficient in subclinical thyroid disease, and FT4 should be tested as well.
When patients present with symptoms suggestive of hyperthyroidism (e.g., weight loss, heat intolerance, and palpitations), the first step clinicians should take is to ascertain biochemical thyrotoxicosis by testing for FT4 and TSH [74]. As most patients with thyrotoxicosis have primary hyperthyroidism, FT4 level will be elevated and TSH level suppressed. The TRAb test can be ordered as Graves' disease is the commonest cause of primary hyperthyroidism. Apart from aiding in the diagnosis of Graves' disease, the magnitude of its elevation can also serve as a prognostic indicator of remission. In pregnant women with Graves' disease or a history thereof, TRAb is also tested, especially during the later stages of gestation, to assess the risk of fetal/neonatal thyrotoxicosis. FT4 and TSH levels should be regularly monitored during medical treatment of hyperthyroidism. It is important to note that FT4 will respond faster than TSH to anti-thyroid therapy. In fact, TSH recovery can lag behind FT4 recovery by several months [74]. Hence, the dose of medication should be titrated according to improvement in FT4 levels during the initial phases of therapy.
Occasionally, patients may have elevated FT4 level and elevated or inappropriately normal TSH level at presentation. While laboratory assay interferences may explain this TFT abnormality in the absence of thyrotoxicosis symptoms, it is important to consider two other differential diagnoses: secondary hyperthyroidism from TSH-secreting pituitary adenoma (TSHoma) and resistance to thyroid hormone-β (RTHβ). Patients with TSHoma have elevated levels of sex hormone-binding globulin, a peripheral tissue marker of thyroid hormone action [75]. In addition, they have increased levels of alpha-subunit, a high alpha-subunit/TSH ratio, and a blunted response to TRH stimulation [76]. Patients with these biochemical findings should undergo an MRI scan of the pituitary gland, which will typically reveal a macroadenoma. An European study (N=43) indicated that with earlier detection because of better awareness and diagnostic modalities, patients with TSHoma respond well to somatostatin analogues [77].
Resistance to thyroid hormone (RTH) is a very rare genetic syndrome that affects the thyroid hormone receptor isoforms β and α [78]. RTHβ needs to be considered in hyperthyroid TFT results. RTHβ, with an estimated prevalence of 1:60,000 live birth, should be considered in patients with unexplained elevated FT4 and unsuppressed TSH levels (inappropriately normal or elevated). Such patients usually (80–90%) have a positive family history (autosomal dominant inheritance), decreased serum FT4/T3 ratio, and normal or exaggerated response to TRH stimulation.
TFTs (FT4 and TSH) should also be ordered for patients who present with symptoms of hypothyroidism (e.g., weight gain, lethargy, and constipation). Patients with primary hypothyroidism will have low FT4 and elevated TSH levels. The most common cause of primary hypothyroidism is iodine deficiency. However, in iodine-replete regions, Hashimoto's thyroiditis is the most common cause; hence, TPO-Ab should also be tested. Other causes include previous neck surgery, radioactive iodine therapy, and over-treatment with anti-thyroid drugs.
Patients with secondary hypothyroidism will have low FT4 and low or inappropriately normal TSH levels. History of brain/pituitary surgery or radiotherapy should be sought. It is important to assess the other anterior pituitary hormones to rule out hypopituitarism before commencing treatment of secondary hypothyroidism. The pituitary panel should include morning adrenocorticotrophic hormone and cortisol, FSH, LH, estradiol/testosterone, growth hormone, and insulin-like growth factor-1, as well as prolactin. RTHα also need to be considered [78]. RTHα is characterized by normal TSH level in the face of low/low-normal T4 but high/high-normal T3 levels, such that the T4:T3 ratio is quite low.
With treatment of primary hypothyroidism with levothyroxine, FT4 level will improve before TSH. In secondary hypothyroidism, TSH level will remain low/low-normal with levothyroxine replacement; hence, FT4 levels alone will need to be monitored. Because of the physiological changes during pregnancy, pregnant women with hypothyroidism will often need to increase their usual levothyroxine dose by 30% [60]. Their thyroid function has to be monitored closely (every four to six weeks) during pregnancy as maternal hypothyroidism is associated with suboptimal obstetric outcomes and poorer fetal neurocognitive development.
Patients with thyroid nodule(s) should undergo TFTs to assess the functional status of the nodule(s). Hyperfunctioning thyroid nodules are usually not malignant. By contrast, Boelaert, et al. [79] have shown that higher TSH levels, even within the normal range, are associated with increased risk of malignancy. Hence, patients with biochemical overt and subclinical hyperthyroidism should undergo radionuclide scanning to confirm the diagnosis of toxic adenoma or multinodular goiter. Patients who are euthyroid or hypothyroid should be considered for fine-needle biopsy of their thyroid nodule(s) to rule out thyroid malignancy, depending on nodule size and ultrasonographic characteristics [25].
Tg and Tg-Ab are instrumental in the follow-up of DTC, which forms the vast majority of all thyroid cancers. For Tg a cutoff of 1 µg/L is generally used [25]. Recombinant TSH (r-TSH) stimulates the secretion of Tg from remnant thyroid tissue and tissue with metastatic cancer. R-TSH-stimulated Tg level is used to ascertain disease remission. However, with the advent of highly sensitive Tg assays (with functional sensitivity <0.1–0.2 µg/L), the use of r-TSH may progressively decline. As mentioned above, the presence of Tg-Ab may lead to falsely low Tg levels; hence, Tg and Tg-Ab must be measured concurrently. The Tg-Ab trend can also be used as a surrogate tumor marker of DTC [25]. Patients with decreasing Tg-Ab levels are at a lower risk of recurrent or persistent disease. TSH is also important in the management of DTC. This is because DTC expresses TSH receptors and hence responds to TSH stimulation. Patients with DTC frequently need supra-physiological doses of levothyroxine postoperatively to intentionally suppress the TSH levels during the initial postoperative period, especially if they have high-risk tumors and are not in remission (i.e., TSH suppression therapy). With control of the disease, the low TSH targets can be gradually increased upwards.
Like Tg in DTC, calcitonin is an important tumor marker of MTC. In the absence of interfering factors, calcitonin levels correlate with the size and volume of MTC. A level of more than 100 pg/mL is virtually diagnostic of MTC; markedly elevated calcitonin above this level at diagnosis indicates distant metastasis [80]. The trend in postoperative calcitonin is used to follow up patients with MTC. A doubling time of less than two yrs generally signifies poor prognosis.
Go to : 
The TSH test is the best initial test for thyroid dysfunction in most patients [147481], and its sensitivity and specificity are superior to those of thyroid hormone tests [82]. FT4 testing may not add to patient management when TSH is normal [83], but it is useful when TSH is <0.05 mIU/L [84]. Evaluation of central hypothyroidism, NTI, treated thyroid disorders, the elderly, and patients on concurrent medications are always challenging.
Institutions face increasing pressures for cost containment and cost-effectiveness, and they have attempted to reduce ordering of TFTs [85]. Some institutions opt to provide the TSH test alone as a first-line test, except when clinically indicated (e.g., treated thyroid disease and suspected/known pituitary disease); FT4 level is measured when TSH level is abnormal [86]. The TSH test as an initial test followed by the FT4 test when TSH level is abnormal is better than the reverse sequence [87]. Henze et al. [88] proposed that the TSH-first strategy can be further refined through widening the TSH reference range of 0.4–4.0 mIU/L by 0.1–0.2 mIU/L at the lower cut-point and by 1–2 mIU/L at the top end, with minimal impact on case detection. They found that applying TSH decision limits of 0.2–6.0 mIU/L in 120,403 subjects would have resulted in a 34% reduction in FT4 testing or 22% if more stringent TSH limits of 0.3–5.0 mIU/L were used. Only 4.2% of TSH values between 0.2 mIU/L and 0.4 mIU/L would not have led to detection of high FT4, the values of which were mostly borderline and unlikely to be clinically relevant. Equally, only 2.5% of TSH values between 4.0 mIU/L and 6.0 mIU/L were associated with low FT4 level, 94% of which were marginal and unlikely to be clinically significant.
Go to : 
Laboratory tests are integral in the management of hyper- and hypothyroidism, thyroid nodules, and thyroid cancer. It is important to understand the caveats and pitfalls in the interpretation of such tests. When the results are discordant, clinicians and laboratorians should factor in possible assay interferences or effects of concurrent medications, and interpret the results according to the clinical setting. Close communication between all members of the care team is vital. With good knowledge of laboratory science and appreciation of medical context, it is not difficult to interpret these tests successfully and accurately.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Thienpont LM, Uytfanghe KV, Poppe K, Velkeniers B. Determination of free thyroid hormones. Best Pract Res Clin Endocrinol Metab. 2013; 27:689–700. PMID: 24094639.
2. Lin DC, Straseski JA, Schmidt RL. The Thyroid Benchmarking Group. Multicenter benchmark study reveals significant variation in thyroid testing in the United States. Thyroid. 2017; 27:1232–1245.
3. Larsen PR. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med. 1982; 306:23–32. PMID: 7031472.
4. Benhadi N, Fliers E, Visser TJ, Reitsma JB, Wiersinga WM. Pilot study on the assessment of the setpoint of the hypothalamus-pituitary-thyroid axis in healthy volunteers. Eur J Endocrinol. 2010; 162:323–329. PMID: 19926783.
5. Brown SJ, Bremner AP, Hadlow NC, Feddema P, Leedman PJ, O'Leary PC, et al. The log TSH-free T4 relationship in a community-based cohort is nonlinear and is influenced by age, smoking and thyroid peroxidase antibody status. Clin Endocrinol (Oxf). 2016; 85:789–796. PMID: 27197788.
6. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Recent advances in thyroid hormone regulation: toward a new paradigm for optimal diagnosis and treatment. Front Endocrinol (Lausanne). 2017; 8:364. PMID: 29375474.
7. Rothacker KM, Brown SJ, Hadlow NC, Wardrop R, Walsh JP. Reconciling the log-linear and non-log-linear nature of the TSH-free T4 relationship: intra-individual analysis of a large population. J Clin Endocrinol Metab. 2016; 101:1151–1158. PMID: 26735261.
8. van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem. 2011; 57:122–127. PMID: 21097676.
9. Caron PJ, Nieman LK, Rose SR, Nisula BC. Deficient nocturnal surge of thyrotropin in central hypothyroidism. J Clin Endocrinol Metab. 1986; 62:960–964. PMID: 3958131.
10. Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocr Rev. 2013; 34:619–657. PMID: 23575764.
11. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002; 87:489–499. PMID: 11836274.
12. Saw S, Sethi S, Aw TC. Technical evaluation of thyroid assays on the Vitros ECi. Clin Chem. 1999; 45:578–580. PMID: 10102924.
13. Brabant G, Beck-Peccoz P, Jarzab B, Laurberg P, Orgiazzi J, Szabolcs I, et al. Is there a need to redefine the upper normal limit of TSH? Eur J Endocrinol. 2006; 154:633–637. PMID: 16645008.
14. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. American association of clinical endocrinologists, American thyroid association taskforce on hypothyroidism in adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the american association of clinical endocrinologists and the American thyroid association. Thyroid. 2012; 22:1200–1235. PMID: 22954017.
15. Esfandiari NH, Papaleontiou M. Biochemical testing in thyroid disorders. Endocrinol Metab Clin North Am. 2017; 46:631–648. PMID: 28760230.
16. Spencer CA, LoPresti JS, Patel A, Guttler RB, Eigen A, Shen D, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab. 1990; 70:453–460. PMID: 2105333.
17. Li H, Yuan X, Liu L, Zhou J, Li C, Yang P, et al. Clinical evaluation of various thyroid hormones on thyroid function. Int J Endocrinol. 2014; 2014:618572. PMID: 25548564.
18. Estrada JM, Soldin D, Buckey TM, Burman KD, Soldin OP. Thyrotropin isoforms: implications for thyrotropin analysis and clinical practice. Thyroid. 2014; 24:411–423. PMID: 24073798.
19. Mammen JS, McGready J, Ladenson PW, Simonsick EM. Unstable thyroid function in older adults is caused by alterations in both thyroid and pituitary physiology and is associated with increased mortality. Thyroid. 2017; 27:1370–1377. PMID: 28854871.
20. Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012; 97:1554–1562. PMID: 22344200.
21. Dufour DR. Laboratory tests of thyroid function: uses and limitations. Endocrinol Metab Clin North Am. 2007; 36:579–594. PMID: 17673120.
22. Cho YY, Song JS, Park HD, Kim YN, Kim HI, Kim TH, et al. First report of familial dysalbuminemic hyperthyroxinemia with an ALB variant. Ann Lab Med. 2017; 37:63–65. PMID: 27834068.
23. Midgley JE. Direct and indirect free thyroxine assay methods: theory and practice. Clin Chem. 2001; 47:1353–1363. PMID: 11468222.
24. Lee MN, Lee SY, Hur KY, Park HD. Thyroxine (T4) autoantibody interference of free T4 concentration measurement in a patient with Hashimoto's thyroiditis. Ann Lab Med. 2017; 37:169–171. PMID: 28029007.
25. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.
26. Giovanella L, Imperiali M, Ferrari A, Palumbo A, Furlani L, Graziani MS, et al. Serum thyroglobulin reference values according to NACB criteria in healthy subjects with normal thyroid ultrasound. Clin Chem Lab Med. 2012; 50:891–893. PMID: 22628333.
27. Spencer CA, Petrovic I, Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2011; 96:1283–1291. PMID: 21325460.
28. Spencer CA. Clinical review: clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011; 96:3615–3627. PMID: 21917876.
29. Netzel BC, Grebe SK, Carranza Leon BG, Castro MR, Clark PM, Hoofnagle AN, et al. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J Clin Endocrinol Metab. 2015; 100:E1074–E1083. PMID: 26079778.
30. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995; 43:55–68. PMID: 7641412.
31. Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002; 87:3221–3226. PMID: 12107228.
32. Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O'Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab. 2010; 95:1095–1104. PMID: 20097710.
33. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of the thyroid disease during pregnancy and postpartum. Thyroid. 2017; 27:315–389. PMID: 28056690.
34. McLachlan SM, Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid. 2013; 23:14–24. PMID: 23025526.
35. Barbesino G, Tomer Y. Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab. 2013; 98:2247–2255. PMID: 23539719.
36. Hermsen D, Broecker-Preuss M, Casati M, Mas JC, Eckstein A, Gassner D, et al. Technical evaluation of the first fully automated assay for the detection of TSH receptor autoantibodies. Clin Chim Acta. 2009; 401:84–89. PMID: 19091299.
37. Tozzoli R, d'Aurizio F, Villalta D, Giovanella L. Evaluation of the first fully automated immunoassay method for the measurement of stimulating TSH receptor autoantibodies in Graves' disease. Clin Chem Lab Med. 2017; 55:58–64. PMID: 27331310.
38. Autilio C, Morelli R, Locantore P, Pontecorvi A, Zuppi C, Carrozza C. Stimulating TSH receptor autoantibodies immunoassay: analytical evaluation and clinical performance in Graves' disease. Ann Clin Biochem. 2018; 55:172–177. PMID: 28388869.
39. Niccoli P, Conte-Devolx B, Lejeune PJ, Carayon P, Henry JF, Roux F, et al. Hypercalcitonemia in conditions other than medullary cancers of the thyroid. Ann Endocrinol (Paris). 1996; 57:15–21. PMID: 8734284.
40. Karanikas G, Moameni A, Poetzi C, Zettinig G, Kaserer K, Bieglmayer C, et al. Frequency and relevance of elevated calcitonin levels in patients with neoplastic and nonneoplastic thyroid disease and in healthy subjects. J Clin Endocrinol Metab. 2004; 89:515–519. PMID: 14764755.
41. Trimboli P, Giovanella L, Crescenzi A, Romanelli F, Valabrega S, Spriano G, et al. Medullary thyroid cancer diagnosis: an appraisal. Head Neck. 2014; 36:1216–1223. PMID: 23955938.
42. Algeciras-Schimnich A, Preissner CM, Theobald JP, Finseth MS, Grebe SKG. Procalcitonin: a marker for the diagnosis and follow-up of patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2009; 94:861–868. PMID: 19088163.
43. Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab. 2014; 99:2986–2994. PMID: 24840813.
44. Trimboli P, Seregni E, Treglia G, Alevizaki M, Giovanella L. Procalcitonin for detecting medullary thyroid carcinoma: a systematic review. Endocr Relat Cancer. 2015; 22:R157–R164. PMID: 25934688.
45. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013; 27:745–762. PMID: 24275187.
46. Samarasinghe S, Meah F, Singh V, Basit A, Emanuele N, Emanuele MA, et al. Biotin interference with routine clinical immunoassays: understand the causes and mitigate the risks. Endocr Pract. 2017; 23:989–998. PMID: 28534685.
47. Piketty ML, Prie D, Sedel F, Bernard D, Hercend C, Chanson P, et al. High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference. Clin Chem Lab Med. 2017; 55:817–825. PMID: 28222020.
48. Kummer S, Hermsen D, Distelmaier F. Biotin treatment mimicking Graves' disease. N Engl J Med. 2016; 375:704–706.
49. Trambas C, Lu Z, Yen T, Sikaris K. Depletion of biotin using streptavidin-coated microparticles: a validated solution to the problem of biotin interference in streptavidin-biotin immunoassays. Ann Clin Biochem. 2018; 55:216–226. PMID: 28406314.
50. Stockigt JR, Lim CF. Medications that distort in vitro tests of thyroid function, with particular reference to estimates of serum free thyroxine. Best Pract Res Clin Endocrinol Metab. 2009; 23:753–767. PMID: 19942151.
51. Barbesino G. Drugs affecting thyroid function. Thyroid. 2010; 20:763–770. PMID: 20578900.
52. Fournier JP, Yin H, Yu OH, Azoulay L. Metformin and low levels of thyroid-stimulating hormone in patients with type 2 diabetes mellitus. CMAJ. 2014; 186:1138–1145. PMID: 25246411.
53. Lupoli R, Di Minno A, Tortora A, Ambrosino P, Lupoli GA, Di Minno MN. Effects of treatment with metformin on TSH levels: a meta-analysis of literature studies. J Clin Endocrinol Metab. 2014; 99:E143–E148. PMID: 24203069.
54. Illouz F, Braun D, Briet C, Schweizer U, Rodien P. Endocrine side-effects of anti-cancer drugs: thyroid effects of tyrosine kinase inhibitors. Eur J Endocrinol. 2014; 171:R91–R99. PMID: 24833135.
55. Dlamini S, Reddy A, Gounden V. How low can thyroid tests go? Clin Chem. 2018; 64:755. PMID: 29592909.
56. Danzi S, Klein I. Amiodarone-induced thyroid dysfunction. J Intensive Care Med. 2015; 30:179–185. PMID: 24067547.
57. Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010; 95:2529–2535. PMID: 20525904.
58. Tomisti L, Rossi G, Bartalena L, Martino E, Bogazzi F. The onset time of amiodarone-induced thyrotoxicosis (AIT) depends on AIT type. Eur J Endocrinol. 2014; 171:363–368. PMID: 24935933.
59. Benjamens S, Dullaart RPF, Sluiter WJ, Rienstra M, van Gelder IC, Links TP. The clinical value of regular thyroid function tests during amiodarone treatment. Eur J Endocrinol. 2017; 177:9–14. PMID: 28424174.
60. Soh SB, Topliss DJ. Thyroid dysfunction in pregnancy: optimising obstetric outcomes. Endocrinology Today. 2013; 4:8–16.
61. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011; 21:1081–1125. PMID: 21787128.
62. Shen FX, Xie ZW, Lu SM, Aw TC, Zhu B. Gestational thyroid reference intervals in antibody-negative Chinese women. Clin Biochem. 2014; 47:673–675. PMID: 24631176.
63. Ong GS, Hadlow NC, Brown SJ, Lim EM, Walsh JP. Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation? J Clin Endocrinol Metab. 2014; E2668–E2672. PMID: 25226292.
64. Vila L, Velasco I, González S, Morales F, Sánchez E, Torrejón S, et al. Controversies in endocrinology: on the need for universal thyroid screening in pregnant women. Eur J Endocrinol. 2014; 170:R17–R30. PMID: 24128429.
65. Stagnaro-Green A. Approach to the patient with postpartum thyroiditis. J Clin Endocrinol Metab. 2012; 97:334–342. PMID: 22312089.
66. Stuckey BG, Kent GN, Ward LC, Brown SJ, Walsh JP. Postpartum thyroid dysfunction and the long-term risk of hypothyroidism: results from a 12-year follow-up study of women with and without postpartum thyroid dysfunction. Clin Endocrinol (Oxf). 2010; 73:389–395. PMID: 20184598.
67. Stuckey BG, Kent GN, Allen JR, Ward LC, Brown SJ, Walsh JP. Low urinary iodine postpartum is associated with hypothyroid postpartum thyroid dysfunction and predicts long-term hypothyroidism. Clin Endocrinol (Oxf). 2011; 74:631–635. PMID: 21470286.
68. Mebis L, Van den Berghe G. Thyroid axis function and dysfunction in critical illness. Best Pract Res Clin Endocrinol Metab. 2011; 25:745–757. PMID: 21925075.
69. van den Berghe G. Non-thyroidal Illness in the ICU: a syndrome with different faces. Thyroid. 2014; 24:1456–1465. PMID: 24845024.
70. Pappa TA, Vagenakis AG, Alevizaki M. The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest. 2011; 41:212–220. PMID: 20964678.
71. Bowers J, Terrien J, Clerget-Froidevaux MS, Gothié JD, Rozing MP, Westendorp RG, et al. Thyroid hormone signaling and homeostasis during aging. Endocr Rev. 2013; 34:556–589. PMID: 23696256.
72. Laurberg P, Andersen S, Carlé A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol. 2011; 7:232–239. PMID: 21301488.
73. Kahapola-Arachchige KM, Hadlow N, Wardrop R, Lim EM, Walsh JP. Age-specific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clin Endocrinol (Oxf). 2012; 77:773–779. PMID: 22703566.
74. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016; 26:1343–1421. PMID: 27521067.
75. Beck-Peccoz P, Persani L, Mannavola D, Campi I. Pituitary tumours: TSH-secreting adenomas. Best Pract Res Clin Endocrinol Metab. 2009; 23:597–606. PMID: 19945025.
76. Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab. 1999; 84:476–486. PMID: 10022404.
77. Socin HV, Chanson P, Delemer B, Tabarin A, Rohmer V, Mockel J, et al. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. Eur J Endocrinol. 2003; 148:433–442. PMID: 12656664.
78. Singh BK, Yen PM. A clinician's guide to understanding resistance to thyroid hormone due to receptor mutations in the TRα and TRβ isoforms. Clin Diabetes Endocrinol. 2017; 3:8. PMID: 28932413.
79. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006; 91:4295–4301. PMID: 16868053.
80. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association Guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015; 25:567–610. PMID: 25810047.
81. Sheehan MT. Biochemical testing of the thyroid: TSH is the best and, oftentimes, only test needed - a review for primary care. Clin Med Res. 2016; 14:83–92. PMID: 27231117.
82. de los Santos ET, Starich GH, Mazzaferri EL. Sensitivity, specificity, and cost-effectiveness of the sensitive thyrotropin assay in the diagnosis of thyroid disease in ambulatory patients? Arch Intern Med. 1989; 149:526–532. PMID: 2493228.
83. Bauer DC, Brown AN. Sensitive thyrotropin and free thyroxine testing in outpatients. Are both necessary? Arch Intern Med. 1996; 156:2333–2337. PMID: 8911240.
84. Ross DS, Daniels GH, Gouveia D. The use and limitations of a chemiluminescent thyrotropin assay as a single thyroid function test in an out-patient endocrine clinic. J Clin Endocrinol Metab. 1990; 71:764–769. PMID: 2394778.
85. Zhelev Z, Abbott R, Rogers M, Fleming S, Patterson A, Hamilton WT, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016; 6:e010065.
86. Ross DS. Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol Metab Clin North Am. 2001; 30:245–264. PMID: 11444162.
87. Nordyke RA, Reppun TS, Madanay LD, Woods JC, Goldstein AP, Miyamoto LA. Alternative sequences of thyrotropin and free thyroxine assays for routine thyroid function testing. Quality and cost. Arch Intern Med. 1998; 158:266–272. PMID: 9472207.
88. Henze M, Brown SJ, Hadlow NC, Walsh JP. Rationalizing thyroid function testing: which TSH cutoffs are optimal for testing free T4? J Clin Endocrinol Metab. 2017; 102:4235–4241. PMID: 28938415.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download