Alzheimer's disease (AD) is characterized by the progressive loss of memory and a decline in intellectual abilities, thus interfering with daily life. The current diagnostic criteria for AD focus on symptoms or signs of thinking, learning, and memory deficits [
1]. The diagnostic accuracy is relatively low, with a specificity of around 70% and a sensitivity of 80%, even in patients undergoing clinical follow-up for several years at expert research centers [
2]. Cerebrospinal fluid (CSF) assays, brain positron emission tomography imaging (PET), and structural magnetic resonance imaging are used for AD diagnosis to enhance the pathophysiological specificity, and specificity has improved to 90% [
34].
Amyloid β (Aβ), a secreted peptide normally present in plasma and CSF, is derived from a large precursor protein via the sequential action of two proteases, i.e., β and γ secretase. Secreted Aβ typically has 40 amino acids (Aβ40); however, a small percentage has 42 amino acids (Aβ42) [
5]. Aβ42 deposits in the brain, and the presence of amyloid-containing senile plaques, together with neurofibrillary tangles, are hallmarks of AD. Aβ42 levels in the CSF are significantly reduced in patients with AD [
6]. CSF evaluation is helpful in AD diagnosis; however, its screening is limited by the invasiveness of the procedure. Plasma is a more accessible and less invasive source than CSF for estimating circulating Aβ concentrations. Although plasma Aβ concentrations are not highly correlated with CSF Aβ concentrations or those determined by PET results [
7], recent studies have shown that a low plasma Aβ42/Aβ40 ratio is associated with greater cognitive decline over time and can be helpful for predicting mild cognitive impairment (MCI) or AD [
89]. Therefore, CSF Aβ or PET amyloid plaque measurements cannot be replaced with analyses of plasma Aβ, though plasma Aβ may have predictive value. However, there is no established cutoff value or reference interval for plasma Aβ. The aim of this study was to establish plasma Aβ reference intervals for Korean adults without cognitive impairment.
This study included 370 Korean adults (aged 40-69 yr) who visited the Health Promotion Center of Ajou University Hospital, Suwon, Korea. Screening of the subjects was based on a complete medical history, physical examination, vital signs, and clinical laboratory tests. The Korean version of the Mini-Mental State Examination (MMSE) was used as a screening tool for cognitive function. In the Korean version of the MMSE, subjects are divided into three groups: definite non-dementia (score of 25 or higher), questionable dementia (score of 21-24), and definite dementia (score of 20 or less) [
10]. Subjects with definite non-dementia were selected in this study. Participants with a history of hypertension, stroke, major depressive disorder, bipolar disorder, schizophrenia, substance abuse disorder, those with known cognitive impairment, reported use of drugs such as donepezil, memantine, galantamine, or cholinergic agents that interfere with cognitive function, and those with a history of traumatic brain injury or other neurological disease were excluded from this study. This study was approved by the Ethics Review Board of Ajou University Medical Center. All participants were provided detailed written and oral information on the study and were required to provide written informed consent prior to screening for eligibility.
Fasting blood was drawn between 8 am and 10 am by using EDTA vacutainer (BD, Franklin Lakes, NJ, USA). The blood samples were put on ice immediately and centrifuged at 1,902g for 10 min at 4℃ to generate plasma. The plasma was then stored in polypropylene tubes at -20℃ and sent to the analytical laboratory within 10 hr of sampling. Repeated freeze/thaw cycles may affect the plasma Aβ concentration; accordingly, all samples were frozen and thawed once. Plasma Aβ40 and Aβ42 levels were measured by using a human amyloid β (N) assay kit (Immuno-Biological Laboratories, Takasaki-shi, Japan) after they were thawed at room temperature. These ELISA kits have been used to quantify levels of Aβ40 and Aβ42 in previous studies [
1112]. Wells were pre-coated with a monoclonal anti-Aβ antibody that was specific to the N-terminal sequence of human Aβ peptide. The detection antibody was specific to the C-terminal sequence of the human Aβ peptide. The plasma Aβ assay was carried out in accordance with the manufacturer's instructions. The mean intra-assay and inter-assay coefficients of variation for Aβ40 and Aβ42 were less than 10%.
Descriptive statistics were calculated by using the demographic data. First, a multiple regression analysis was conducted by using stepwise selection methods. The associations of sex, age, body mass index, and other demographic characteristics with plasma Aβ concentrations were analyzed. Estimates of the reference interval were obtained according to the Clinical and Laboratory Standards Institute guidelines for defining, establishing, and verifying reference intervals in the clinical laboratory (EP28-A3c) [
13]. The need for gender or age partitioning was evaluated by using the Harris and Boyd method [
13]. Reference intervals were then calculated by using non-parametric methods, and the lower and upper limits represented the 2.5th and 97.5th percentiles. Statistical analysis was performed by using SPSS version 12.0 (SPSS Korea, Seoul, Korea).
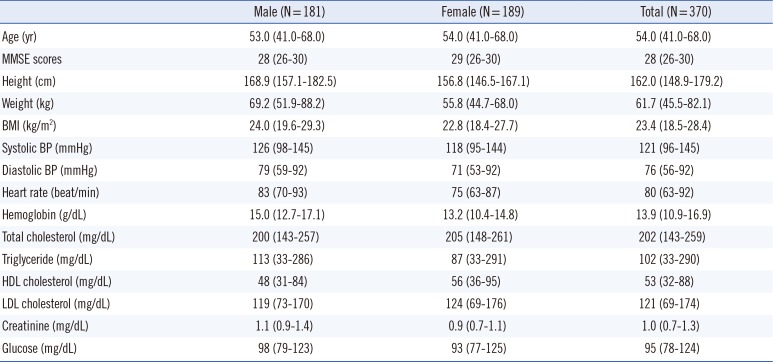
A total of 181 male (48.9%) and 189 female (51.1%) subjects aged 40-69 yr were enrolled in the study (
Table 1). Based on the demographic and baseline characteristics, most subjects were apparently healthy. All subjects scored ≥26 on the MMSE. Given that Aβ40, Aβ42, and the Aβ42/Aβ40 ratio did not fit a Gaussian distribution, log-transformed data were used in the multiple regression analysis. Sex and age were statistically significant variables with respect to Aβ40 and Aβ42 (
Supplemental Data Table S1). However, based on an analysis using the Harris and Boyd method, partitioning the data by sex or age group was not necessary. Therefore, reference intervals were developed without partitioning. The distributions of Aβ40, Aβ42, and the Aβ42/Aβ40 ratio are shown in
Fig. 1, and the reference intervals were calculated by using non-parametric methods. The median concentrations and the 2.5th to 97.5th percentile values of Aβ40, Aβ42, and Aβ42/Aβ40 are presented in
Table 2. The 95th percentile reference intervals for Aβ40 and Aβ42 were 127-331 pg/mL and 2.31-19.84 pg/mL. The reference interval for the Aβ42/Aβ40 ratio was 0.011-0.092.
Fig. 1
Distribution of amyloid β40 (A), amyloid β42 (B), and the ratio of amyloid β42 to amyloid β40 (C). Dotted lines indicate lower and upper reference limits.
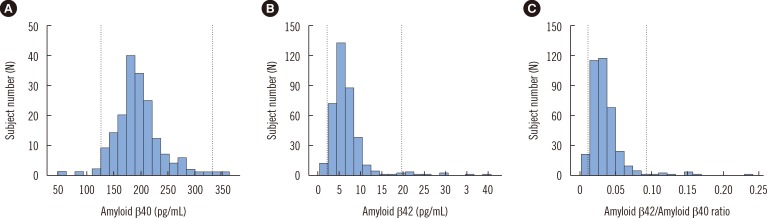

Table 1
Subject characteristics
|
Male (N = 181) |
Female (N = 189) |
Total (N = 370) |
|
Age (yr) |
53.0 (41.0–68.0) |
54.0 (41.0–68.0) |
54.0 (41.0–68.0) |
|
MMSE scores |
28 (26–30) |
29 (26–30) |
28 (26–30) |
|
Height (cm) |
168.9 (157.1–182.5) |
156.8 (146.5–167.1) |
162.0 (148.9–179.2) |
|
Weight (kg) |
69.2 (51.9–88.2) |
55.8 (44.7–68.0) |
61.7 (45.5–82.1) |
|
BMI (kg/m2) |
24.0 (19.6–29.3) |
22.8 (18.4–27.7) |
23.4 (18.5–28.4) |
|
Systolic BP (mmHg) |
126 (98–145) |
118 (95–144) |
121 (96–145) |
|
Diastolic BP (mmHg) |
79 (59–92) |
71 (53–92) |
76 (56–92) |
|
Heart rate (beat/min) |
83 (70–93) |
75 (63–87) |
80 (63–92) |
|
Hemoglobin (g/dL) |
15.0 (12.7–17.1) |
13.2 (10.4–14.8) |
13.9 (10.9–16.9) |
|
Total cholesterol (mg/dL) |
200 (143–257) |
205 (148–261) |
202 (143–259) |
|
Triglyceride (mg/dL) |
113 (33–286) |
87 (33–291) |
102 (33–290) |
|
HDL cholesterol (mg/dL) |
48 (31–84) |
56 (36–95) |
53 (32–88) |
|
LDL cholesterol (mg/dL) |
119 (73–170) |
124 (69–176) |
121 (69–174) |
|
Creatinine (mg/dL) |
1.1 (0.9–1.4) |
0.9 (0.7–1.1) |
1.0 (0.7–1.3) |
|
Glucose (mg/dL) |
98 (79–123) |
93 (77–125) |
95 (78–124) |

Table 2
Reference intervals for amyloid β40, amyloid β42, and the ratio of amyloid β42 to amyloid β40 (N=370)
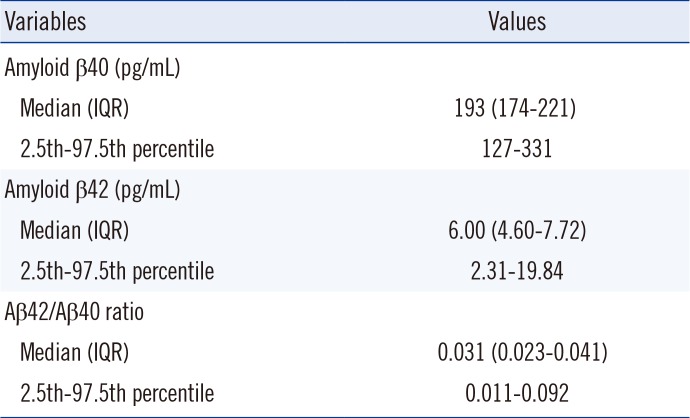
|
Variables |
Values |
|
Amyloid β40 (pg/mL) |
|
|
Median (IQR) |
193 (174–221) |
|
2.5th–97.5th percentile |
127–331 |
|
Amyloid β42 (pg/mL) |
|
|
Median (IQR) |
6.00 (4.60–7.72) |
|
2.5th–97.5th percentile |
2.31–19.84 |
|
Aβ42/Aβ40 ratio |
|
|
Median (IQR) |
0.031 (0.023–0.041) |
|
2.5th–97.5th percentile |
0.011–0.092 |

The reference intervals for Tau and Aβ42 in CSF obtained from healthy adults have been previously reported [
1415]; however, to the best of our knowledge, this is the first study presenting reference intervals for plasma Aβ40, Aβ42, and Aβ42/Aβ40 based on a large sample of individuals without cognitive impairment. There is an established relationship between neurotoxic Aβ and AD [
56]; furthermore, concentrations of Aβ42 in CSF are a reliable diagnostic biomarker for AD [
6]. Plasma Aβ does not originate only in the brain; it is also the product of amyloid precursor protein (APP) metabolism in the skeletal muscle, pancreas, kidney, liver, vascular walls, lung, intestine, skin, and several glands [
16]. Platelets are another important source of Aβ, and activated platelets release APP and Aβ [
17]. The blood-brain barrier and the blood-CSF barrier regulate the passage of Aβ between blood and the central nervous system. Therefore, plasma Aβ has not been considered a diagnostic biomarker for AD in cross-sectional studies. Recently, some prospective studies have suggested that a low plasma Aβ42/Aβ40 ratio is associated with greater cognitive decline over time and may be a useful premorbid biomarker for identifying elderly subjects with normal cognitive function that are at an increased risk for developing MCI or AD [
89]. Consequently, Aβ reference intervals are required.
The present study had several strengths. We used the Korean version of MMSE as a screening tool for cognitive function, and subjects who scored 26 or higher were enrolled. In addition, we controlled for various potential confounders, such as history of hypertension, stroke, major depressive disorder, bipolar disorder, schizophrenia, substance abuse disorder, and the use of drugs that interfere with cognitive function, by establishing strict inclusion/exclusion criteria. Finally, reference intervals were derived from a sufficient number of subjects, according to the CLSI guidelines.
The results of this study were limited by the inclusion of adult subjects aged 40-69 yr only. Therefore, the study results cannot be generalized to all elderly populations. Second, the Aβ42 assay kit was intended for CSF Aβ42 measurements; therefore, low levels of plasma Aβ42 may not be accurately quantified by using this assay. In addition, we were unable to collect information about the educational status of subjects. Although all subject scored 26 or higher in the Korean version of the MMSE, it is possible that highly educated MCI subjects were included in this study. In a previous study, plasma Aβ40, Aβ42, and Aβ42/Aβ40 were 197.01 pg/mL, 9.86 pg/mL, and 0.10, respectively, in middle-aged (39-56 yr) normal controls (Human β Amyloid ELISA kit, Wako) [
18]. Aβ42 levels were higher than those in this study. There may be inter-laboratory variability related to both analytical procedures and kit-to-kit variation.
In conclusion, we established reference intervals for plasma Aβ40, Aβ42, and the Aβ42/Aβ40 ratio, in Korean individuals without cognitive impairment. Further studies are needed to define a clear cutoff value for the identification of high-risk individuals, years before clinical symptoms are evident.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download