Abstract
Background
To rapidly obtain outpatient results, we use plasma separation tubes (PST) for chemistry analysis. If lactate dehydrogenase measurement is required, serum separation tubes (SST) are used. There has been no evaluation of hemolysis with these tubes. We compared the hemolytic index (HI) obtained by using PST and SST and applied this for choosing appropriate tubes for clinical laboratories.
Methods
The HI of specimens obtained from outpatients visiting Asan Medical Center between July and December 2012 was analyzed. The HI was scored from 0 to 10 by using the Toshiba 200FR (Toshiba Medical Systems Co., Japan). HI was classified by sample tube type, and significant hemolysis was defined as a HI of 2 or more. For significant hemolysis cases, medical records were reviewed to identify the causes.
Results
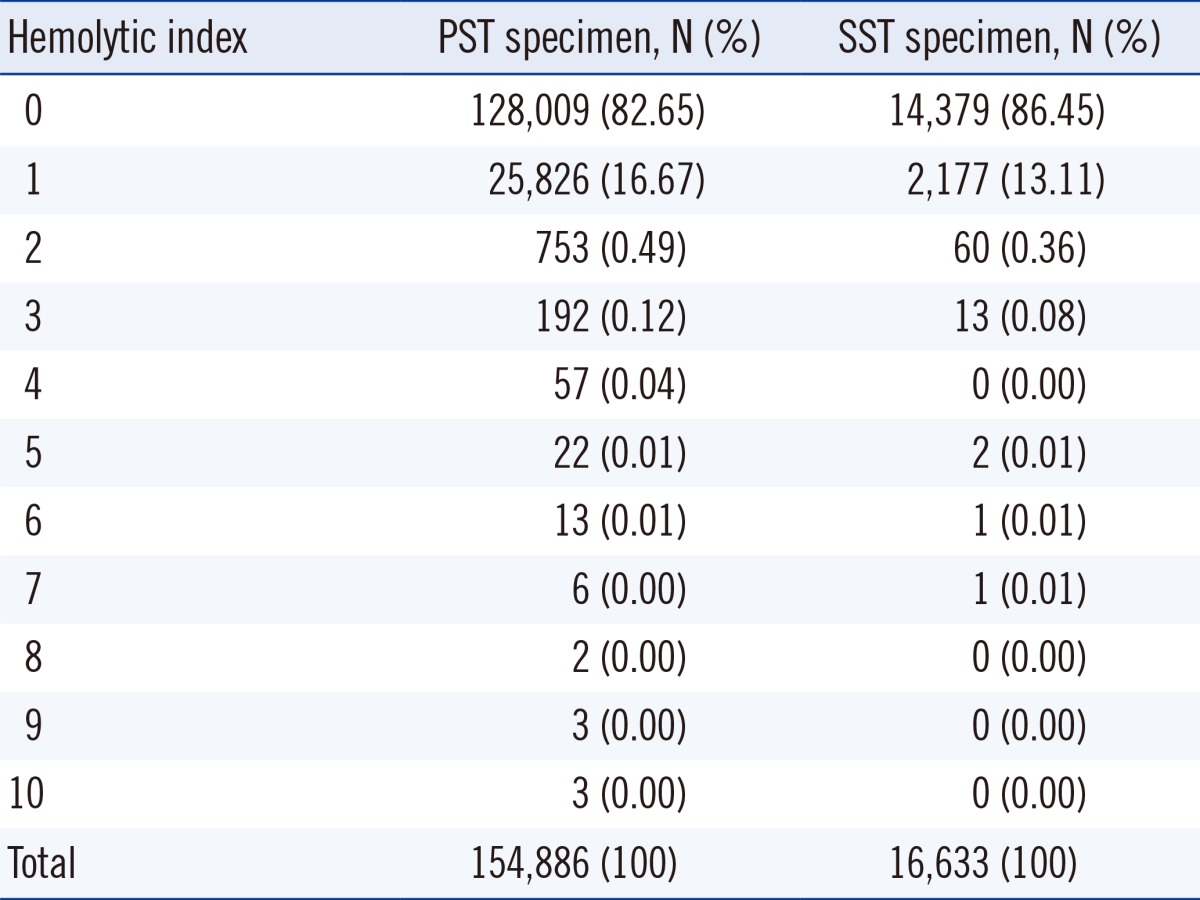
Among 171,519 specimens, significant hemolysis was observed in 0.66% of specimens (0.68% of PST specimens, 0.46% of SST specimens). The mean HI in PST was 0.18 (SD: 0.43) and that in SST was 0.14 (SD: 0.37). The proportion of significant hemolysis was significantly higher in PST than in SST (P=0.001). The cause of significant hemolysis was identified as chemotherapy and prosthetic valve in 48.1% of specimens. Complex sampling errors may have caused significant hemolysis in the remaining 51.9% of specimens.
At our institution, test results for outpatients are provided within 1 hr of sampling [1]. To reduce the turnaround time (TAT) for general chemistry analysis, plasma separation tubes (PST, Greiner Bio-One, Austria) are used, which are not dependent on coagulation. When lactate dehydrogenase (LD) testing is requested, serum separation tubes (SST, Greiner Bio-One) are used, as the results for serum differ from those for plasma [2].
Hemolysis is one of the most common preanalytical variables to affect test results. There are three mechanisms through which red blood cell (RBC) hemolysis may affect test results. The first is the difference between the intracellular and extracellular RBCs concentrations. A previous study showed that in samples with low levels of hemolysis (free Hb less than 0.6 g/L), LD and potassium were increased by 24% and 3.2%, respectively [3]. In contrast, a dilution effect has been observed for albumin in the presence of hemolysis. The second mechanism through which RBC hemolysis may affect test results is optical interference caused by the color of Hb. The third mechanism is the interference of materials from RBCs with reactions [4]. Although not yet standardized, the hemolytic index (HI) has been suggested as a marker to estimate specimen quality [5, 6, 7].
Several studies have compared test results obtained with PST and SST [8, 9, 10, 11, 12]. However, there has been no comparison of RBC hemolysis between these two types of tubes. In this study, we semi-quantitatively evaluated RBC hemolysis with PST and SST and investigated the cause of significant hemolysis.
We analyzed the HI of the specimens from outpatients who visited the Asan Medical Center (Seoul, Korea) between July 1 and December 31, 2012 and who underwent routine chemistry tests. During the research period, 171,519 specimens were submitted to the laboratory for testing. PST and SST were used for 154,886 and 16,633 specimens, respectively. The HI is a semiquantitative index measured by using the Toshiba-200FR automated instrument (Toshiba Medical Systems Co., Tokyo, Japan). This system reports HI values based on Hb concentration: 0, Hb is not detected; 1, less than 0.5 g/L Hb; 2, 0.5-1.0 g/L Hb; 3, 1.0-1.5 g/L Hb; 4, 1.5-2.0 g/L Hb; 5, 2.0-2.5 g/L Hb; 6, 2.5-3.0 g/L Hb; 7, 3.0-3.5 g/L Hb; 8, 3.5-4.0 g/L Hb; 9, 4.0-4.5 g/L Hb; and 10, more than 4.5 g/L Hb. A free Hb concentration of more than 0.5 g/L affects the direct bilirubin result, and a free Hb concentration of more than 2.5 g/L affects the results of the creatine kinase test. The results of routine chemistry analyses, including AST, ALT, potassium, sodium, and LD tests, may be affected by a free Hb concentration of more than 5 g/L [9]. As a HI of 1 was defined by a free Hb concentration of less than 0.5 g/L and a HI of 2 was defined by a free Hb concentration of 0.5-1.0 g/L, a significant hemolysis was defined as a HI of 2 or more.
When a significant hemolysis was identified, we reviewed the sampling process and the patient's medical records to determine the cause of hemolysis. The causes of RBC hemolysis were classified as in vitro or in vivo. In vitro causes of hemolysis included contamination, incorrect needle size, improper tube mixing, incorrectly filled tube, excessive suction, prolonged storage, difficult collection, and mechanical blood processing. Several intrinsic diseases, including hereditary spherocytosis, paroxysmal nocturnal hemoglobinuria, glucose-6-phosphate dehydrogenase deficiency, thalassemia, and sickle cell disorder, and extrinsic factors, such as chemical agents, physical agents, infectious agents, and immune hemolytic anemia, were considered to be in vivo causes of hemolysis [13, 14]. And the patients under 5 yr old were analyzed separately because we use scalp needles for these patients.
A total of 83.02% (142,388 of 171,591) of specimens had a HI value of 0 and 16.33% (28,003 of 171,519) had a HI value of 1. The average HI value was 0.18 (SD: 0.43) for PST specimens and 0.14 (SD: 0.37) for SST specimens, indicating that the HI was slightly higher for PST than SST specimens. The distribution of HI in each tube is shown in Table 1.
It is difficult to draw blood from children. Samples from young children are more vulnerable to hemolysis.
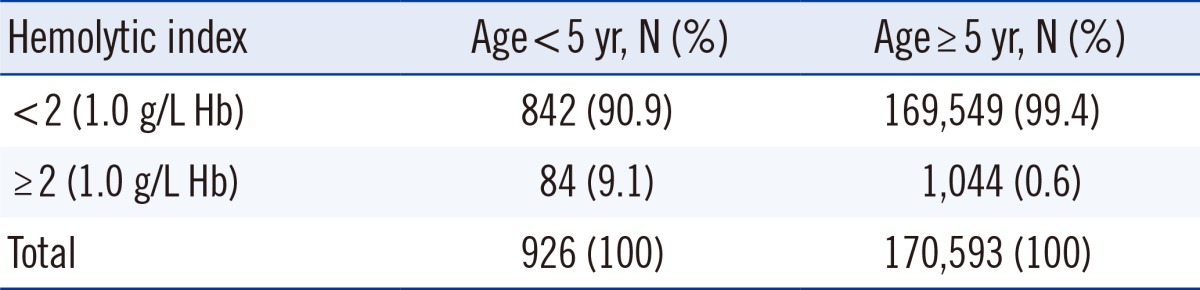
Significant hemolysis occurred more frequently in specimens from young patients (P<0.001, Table 2). As in vivo causes of hemolysis were not identified in the patients under 5 yr old, hemolysis was thought to be due to complex sampling errors.
The medical departments that showed the highest incidences of significant hemolysis were the pediatric chest surgery (8.47%), pediatric hemato-oncology (7.06%), neonatology (4.35%), pediatric urology (2.94%), and pediatric and adolescent medicine (2.63%) departments.
Of the samples that had significant hemolysis (HI≥2), 491 specimens were from patients undergoing chemotherapy (PST, 423; SST, 68), and 52 were from patients with prosthetic valves (PST, 51; SST, 1). The cause of hemolysis was not identified in 558 cases (51.9%).
In order to rapidly report laboratory results, it is important to select appropriate sampling tubes. SST require more time to perform tests because coagulation needs to occur. The TAT for SST is increased, particularly for patients undergoing anticoagulant therapy or those with chronic liver disease [9, 10, 11]. As PST do not require coagulation to occur, they can be centrifuged immediately, reducing the time for analysis. To date, several comparative studies on the test results obtained using PST and SST have been performed [8, 9, 10, 11, 12]; however, no study has compared these types of tubes and the effects of RBC hemolysis.
The mean HI and proportion of significant hemolysis were slightly higher for the PST than for the SST. The higher proportion of hemolysis observed with the use of PST is likely attributable to the continuous friction between RBCs that occurs during centrifugation, whereas specimens collected in SST undergo coagulation prior to centrifugation. It has been demonstrated previously that elevated potassium concentrations occur more frequently with the use of PST than the use of SST [8]. Furthermore, potassium concentration is thought to be increased by hemolysis during specimen transportation [15].
The proportion of specimens that had clear and unclear causes of significant hemolysis were 48.14% (543 of 1,128) and 51.86% (585 of 1,128), respectively. The most frequent cause of significant hemolysis was chemotherapy (PST, 423; SST, 68). Anemia is frequently present in patients undergoing chemotherapy, which may be explained by decreased hematopoiesis or increased hemolysis [16]. It has been reported that a patient receiving 1,3-bis(2-chloroethyl)-1-nitrosourea was vulnerable to hemolysis and showed decreased glutathione reductase levels [17]. Increased mechanical hemolysis has been reported in patients with prosthetic valves [18]; in the current study, 52 cases of hemolysis were caused by prosthetic valves (PST, 51; SST, 1). In the patients included in the study, there were no cases of hereditary spherocytosis, paroxysmal nocturnal hemoglobinuria, glucose-6-phosphate dehydrogenase deficiency, thalassemia, or sickle cell disorder, all of which can trigger in vivo and intrinsic hemolysis. Furthermore, no patients were diagnosed with an infectious agent that causes hemolysis or immune hemolytic anemia.
Cases with known causes of hemolysis were excluded from further analysis. The medical record and sampling process were reviewed for the remaining specimens that had significant hemolysis. No specific causes of hemolysis were identified for 585 cases, which may be as a result of hemolysis being caused by a combination of factors. A total of 84 cases of unknown cause of hemolysis occurred in children younger than 5 yr old. It is difficult to obtain blood specimens from children using a vacuum extraction system because of their thin blood vessels. We used a scalp needle to obtain blood samples from children younger than 5 yr old. Thus, the in vitro hemolysis observed in children younger than 5 yr old was likely caused by multiple factors, including the use of incorrect needle size, excessive suction, and difficult collection. The proportion of cases with HI of 2 or more in children younger than 5 yr old was 9.3%, which is 15-fold higher than that in patients older than 5 yr (0.6%), confirming that hemolysis occurs frequently in children during sampling. Comparison of the departments that had the highest incidence of significant hemolysis revealed that the top five were departments related to children.
There are limitations in this study. It was not possible to take specimens in the two tube types simultaneously from a single patient to directly compare the results in each tube. Thus, individual patient characteristics might affect the results of this study. It was not possible to estimate the effect of hemolysis on chemistry test results.
Specimens collected in SST need coagulation to occur prior to centrifugation. Incomplete clotting can result in fibrin strands in the specimens and critical random errors, which are especially problematic for patients who take anticoagulants or who have chronic liver diseases. This potential risk was considered bigger than the risk from hemolysis, which occurs more frequently with PST than with SST. In addition, PST has a more rapid TAT than SST. Thus, we have decided to continue using PST in our laboratory.
This is the first study to compare the HI of PST and SST specimens. This study will be useful for laboratories identifying the appropriate sample tubes for use in chemistry testing.
References
1. Chung HJ, Lee W, Chun S, Park HI, Min WK. Analysis of turnaround time by subdividing three phases for outpatient chemistry specimens. Ann Clin Lab Sci. 2009; 39:144–149. PMID: 19429800.
2. Bais R, Philcox M. IFCC methods for the measurement of catalytic concentration of enzymes. Part 8. IFCC method for lactate dehydrogenase (L-lactate: NAD oxidoreductase, EC 1.1.1.27). J Automat Chem. 1994; 16:167–182. PMID: 18924988.
3. Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006; 44:311–316. PMID: 16519604.

4. Guder WG, Narayanan S, editors. Samples: From the Patient to the Laboratory: the impact of preanalytical variables on the quality of laboratory results. 3rd ed. Weinheim: Wiley-VCH Verlag GmbH & Co;2003.
5. Lippi G, Luca Salvagno G, Blanckaert N, Giavarina D, Green S, Kitchen S, et al. Multicenter evaluation of the hemolysis index in automated clinical chemistry systems. Clin Chem Lab Med. 2009; 47:934–939. PMID: 19548845.

6. Plebani M, Lippi G. Hemolysis index: quality indicator or criterion for sample rejection? Clin Chem Lab Med. 2009; 47:899–902. PMID: 19642858.

7. Soderberg J, Jonsson PA, Wallin O, Grankvist K, Hultdin J. Haemolysis index--an estimate of preanalytical quality in primary health care. Clin Chem Lab Med. 2009; 47:940–944. PMID: 19589105.
8. Babic N, Zibrat S, Gordon IO, Lee CC, Yeo KT. Effect of blood collection tubes on the incidence of artifactual hyperkalemia on patient samples from an outreach clinic. Clin Chim Acta. 2012; 413:1454–1458. PMID: 22698439.

9. Boyanton BL Jr, Blick KE. Stability studies of twenty-four analytes in human plasma and serum. Clin Chem. 2002; 48:2242–2247. PMID: 12446483.

10. Dimeski G, Masci PP, Trabi M, Lavin MF, de Jersey J. Evaluation of the Becton-Dickinson rapid serum tube: does it provide a suitable alternative to lithium heparin plasma tubes? Clin Chem Lab Med. 2010; 48:651–657. PMID: 20218902.

11. O'Keane MP, Cunningham SK. Evaluation of three different specimen types (serum, plasma lithium heparin and serum gel separator) for analysis of certain analytes: clinical significance of differences in results and efficiency in use. Clin Chem Lab Med. 2006; 44:662–668. PMID: 16681442.
12. Sevastos N, Theodossiades G, Efstathiou S, Papatheodoridis GV, Manesis E, Archimandritis AJ. Pseudohyperkalemia in serum: the phenomenon and its clinical magnitude. J Lab Clin Med. 2006; 147:139–144. PMID: 16503244.

13. Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem. 2000; 46:306–307. PMID: 10657399.

14. Tabbara IA. Hemolytic anemias. Diagnosis and management. Med Clin North Am. 1992; 76:649–668. PMID: 1578962.

15. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005; 46:746–748. PMID: 16183430.

16. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999; 91:1616–1634. PMID: 10511589.

17. Frischer H, Ahmad T. Severe generalized glutathione reductase deficiency after antitumor chemotherapy with BCNU" [1,3-bis(chloroethyl)-1-nitrosourea]. J Lab Clin Med. 1977; 89:1080–1091. PMID: 870569.
18. Brodeur MT, Sutherland DW, Koler RD, Starr A, Kimsey JA, Griswold HE. Red blood cell survival in patients with aortic valvular disease and ball-valve prostheses. Circulation. 1965; 32:570–581. PMID: 5825548.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download