Peritonitis is a potentially serious complication in patients undergoing continuous ambulatory peritoneal dialysis (CAPD). More than 50% of positive cultures from these infections are due to gram-positive bacteria, most commonly coagulase-negative staphylococci and Staphylococcus aureus [1]. Fungal peritonitis accounts for only 3-6% of cases, in which peritoneal dialysis complications are reported [2]. Peritonitis resulting from fungal infection is associated with a longer duration of illness and greater frequencies of hospitalization, high morbidity and mortality rates ranging 20-30% [2], and permanent hemodialysis compared with cases of non-fungal peritonitis [3]. The most common cause of the disease is yeast, with Candida species accounting for 70-90% of infections in adults [2]. We report the first case of CAPD-related peritonitis caused by Cryptococcus arboriformis, an organism never previously been reported as a human pathogen and confirmed by ribosomal DNA gene sequencing.
A 58-yr-old man was admitted complaining of turbid peritoneal dialysis effluent. The patient presented with diabetes mellitus, hypertension, and end-stage renal disease and had been undergoing CAPD for 10 months. He was a heavy drinker, living alone in low socioeconomic conditions, and had not been properly maintaining the cleanliness of his catheter. He had experienced previous episodes of peritonitis, the last occurring 1 month earlier, at which time he was admitted to our hospital. Initially, he was given cefazolin and ceftazidime, administered intraperitoneally, which had no effect. Culture results revealed Aeromonas hydrophila, and the patient was treated with imipenem for 2 weeks, which effectively reduced the white blood cell (WBC) count (8×106 cells/L in peritoneal fluid). He was discharged after 19 days with no symptoms and with a clear dialysate.
On follow-up a week later, the patient again complained of continuous abdominal pain and turbid dialysis effluent. On physical examination, he was febrile, with a healthy catheter exit site. The peritoneal fluid was straw colored and cloudy with WBC count of 1.3×109/L, of which 98% were polymorphonuclear cells and 2% were lymphocytes, confirming peritonitis. Hemoglobin concentration was 7.6 g/dL, WBC count was 9.7×109/L, and platelet count was 339×109/L. Erythrocyte sedimentation rate was 83 mm/hr, C-reactive protein concentration was 1.63 mg/dL, and blood urea nitrogen and creatinine concentrations were 62.2 mg/dL and 8.6 mg/dL, respectively.
The patient was initially treated with cefazolin and ceftazidime, administered intraperitoneally, which produced no clinical improvement. A few days later, culture of the peritoneal fluid revealed an unidentified yeast species, which was also seen on Gram staining of the peritoneal fluid. The patient was readmitted, and oral administration of fluconazole (200 mg/day) was initiated for empirical treatment of CAPD fungal peritonitis. The drug was administered from days 2 to 8. The catheter in his abdomen was removed on day 6, and he was switched to hemodialysis with placement of an appropriate catheter. Despite treatment with antifungal medication and removal of the peritoneal catheter, the patient continued to experience mild fever and abdominal pain, and yeast was again found in the peritoneal fluid culture. Treatment was switched to intravenous amphotericin B (0.5 mg/kg of body weight/day) for 4 weeks, which produced no adverse effects, such as hypersensitivity reactions, and resulted in an improvement of the clinical prognosis. Oral fluconazole (400 mg/day) was administrated for 3 weeks, and the patient was discharged without any symptoms.
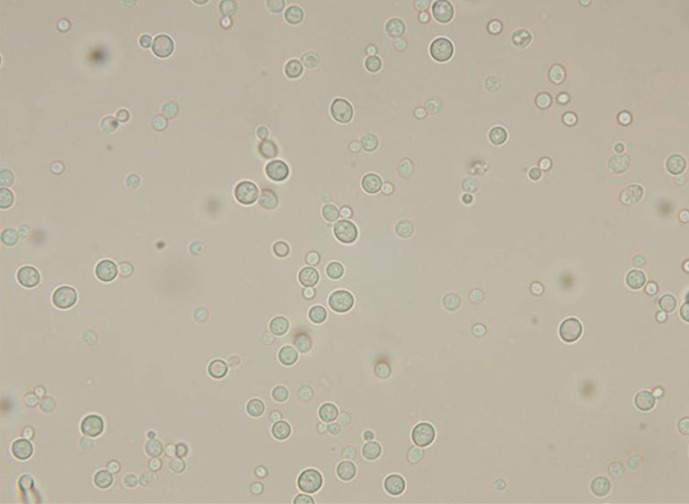
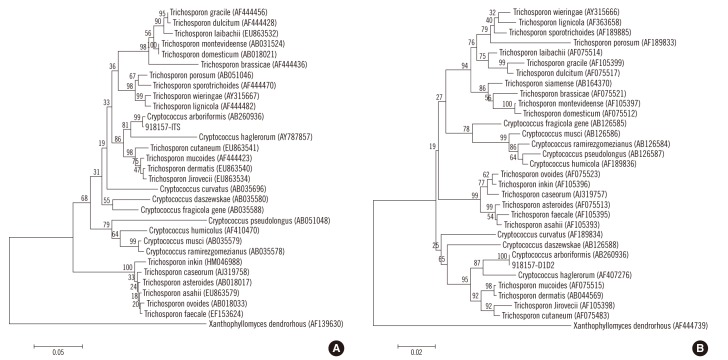
A species of yeast was isolated from peritoneal fluid after culturing the fluid for 48 hr at 35℃ in 5% CO2 on blood agar and chocolate agar plates. The 2- to 3-mm sized colonies that grew on the agar were smooth and white. A wet smear of the peritoneal fluid revealed yeast (Fig. 1), but the isolate was not identified by Vitek 2 YST system (bioMérieux, St. Louis, MO, USA). We performed sequencing of the internal transcribed spacer 1 (ITS1) regions and D1/D2 region of the 28S ribosomal DNA gene; analysis showed 99% homology with Cryptococcus arboriformis ITS1 (GenBank accession no. AB260936) and 100% homology with Cryptococcus arboriformis D1/D2 (Fig. 2). This isolate was ultimately confirmed as Cryptococcus arboriformis through sequence analysis of the 28S ribosomal DNA gene. We performed antifungal susceptibility testing with Vitek 2 AST-YS01 system (bioMérieux). The minimum inhibitory concentrations were 0.5 µg/mL of amphotericin B, ≤1 µg/mL of fluconazole, and 8 µg/mL of flucytosine.
Fungal peritonitis is a severe form of peritonitis that usually occurs after a course of antibiotics prescribed to treat bacterial peritonitis [4]. Predisposing risk factors may include prolonged use of antibiotics, previous bacterial peritonitis, immunosuppression, bowel perforation, malnutrition, and diverticulitis [2]. The effects of comorbid diseases, such as diabetes and cancer, are controversial, and duration of dialysis, age, and sex do not seem to have significant associations with the condition [5, 6]. The present patient had experienced previous bacterial peritonitis, which was treated using broad spectrum antibiotics, including imipenem. His heavy drinking, low socioeconomic status, and unclean catheter may also have predisposed him to the infection [3].
Cryptococcal peritonitis complicating CAPD is rarely reported [7]. A PubMed search, performed by using a combination of the criteria "Cryptococcus," "cryptococcosis," or "cryptococcal" with "peritonitis", produced a list of only 45 cases in the literature, occurring between 1973 and 2011. All but 2 of the isolates were identified as Cryptococcus neoformans, the exceptions being 2 cases of Cryptococcus laurentii [8, 9].
Cryptococcus arboriformis, a basidiomycetous yeast species, was recently isolated from the urine of a Japanese patient with chronic renal failure [10]. It is closely related to Cryptococcus haglerorum and is classified under the Trichosporonales lineage [10]. We were not able to identify our isolate using conventional yeast identification tests because this newly identified species are not yet in the NCBI database (a genome database of the National Center for Biotechnology Information). We identified this strain as Cryptococcus arboriformis on the basis of phylogenetic analysis by sequencing the ITS1 regions and the D1/D2 region of the 28S ribosomal DNA gene.
Previous reported cases of cryptococcal peritonitis associated with CAPD were usually treated with a 2- to 3-month course of antifungal agents and removal of the dialysis catheter [7]. In this case, when the disease was confirmed to be the result of infection by an unidentified yeast species, we suspected a Candida infection, as this organism is the most common pathogen in fungal peritonitis and consequently, we initially treated the patient with fluconazole. Cryptococcus arboriformis was identified after the therapy was changed to amphotericin B. Removal of the catheter was recommended to the patient on day 6, immediately following identification of fungi and continuous administration of antifungal agents for an additional 10 days [11]. A recent study supports catheter removal within 24 hr after the diagnosis of fungal peritonitis [12]. In this case, fluconazole therapy produced no clinical improvement; therefore, the catheter was removed and hemodialysis was begun while continuing the antifungal treatment. Recently, new triazole compounds such as voriconazole and posaconazole have been tested [2]. In a retrospective study, a triazole-flucytosine combination appeared to be as effective as amphotericin B [13]. Echinocandins, such as caspofungin, micafungin, and anidulafungin, are also providing new treatment options for fungal peritonitis, but they are not effective against infections caused by basidiomycetes, such as Cryptococcus [2, 14]. There is a report that ciprofloxacin may be useful as an adjunctive agent in the treatment of cryptococcal peritonitis [15].
In this case, susceptibility testing for some antifungal agents was carried out. The isolate was susceptible to amphotericin B and fluconazole with intermediate sensitivity to flucytosine. As there were no interpretive minimum inhibitory concentration breakpoints available for the isolate, we used the guidelines for Cryptococcus neoformans. Identifying more antifungal agents and establishing a standard for susceptibility tests would be valuable for the treatment of Cryptococcus arboriformis in the future.
Identification of Cryptococcus arboriformis and susceptibility testing were performed after the initial antifungal therapy had been administered. Efficient diagnosis of fungal peritonitis is crucial, if biological or clinical abnormalities continue following treatment for bacterial peritonitis, especially in patients with a history of prolonged use of antibiotics or previous bacterial peritonitis. When an unidentified organism is suspected of causing a disease and conventional identifying methods fail, identification of the pathogen using sequencing and testing the sensitivity of the organism to known antimicrobial agents may lead to an early diagnosis and a better prognosis for patients.
In summary, we describe a case of cryptococcal peritonitis in a patient undergoing CAPD. To the best of our knowledge, this is the first reported case of peritonitis caused by Cryptococcus arboriformis.
References
1. Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002-2003. Perit Dial Int. 2009; 29:297–302. PMID: 19458302.

2. Matuszkiewicz-Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int. 2009; 29(Suppl 2):S161–S165. PMID: 19270208.

3. Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int. 2009; 76:622–628. PMID: 19516241.

4. Nikitidou O, Liakopoulos V, Kiparissi T, Divani M, Leivaditis K, Dombros N. Peritoneal dialysis-related infections recommendations: 2010 update. What is new? Int Urol Nephrol. 2012; 44:593–600. PMID: 21744125.

5. Indhumathi E, Chandrasekaran V, Jagadeswaran D, Varadarajan M, Abraham G, Soundararajan P. The risk factors and outcome of fungal peritonitis in continuous ambulatory peritoneal dialysis patients. Indian J Med Microbiol. 2009; 27:59–61. PMID: 19172063.

6. Rosa NG, Silva S, Lopes JA, Branco P, de Almeida E, Ribeiro C, et al. Fungal peritonitis in peritoneal dialysis patients: Is previous antibiotic therapy an essential condition? Mycoses. 2007; 50:79–81. PMID: 17302754.

7. Yinnon AM, Solages A, Treanor JJ. Cryptococcal peritonitis: report of a case developing during continuous ambulatory peritoneal dialysis and review of the literature. Clin Infect Dis. 1993; 17:736–741. PMID: 8268358.

8. Mocan H, Murphy AV, Beattie TJ, McAllister TA. Fungal peritonitis in children on continuous ambulatory peritoneal dialysis. Scott Med J. 1989; 34:494–496. PMID: 2799371.

9. Sinnott JT 4th, Rodnite J, Emmanuel PJ, Campos A. Cryptococcus laurentii infection complicating peritoneal dialysis. Pediatr Infect Dis J. 1989; 8:803–805. PMID: 2594458.

10. Sugita T, Takashima M, Sano A, Nishimura K, Kinebuchi T, Yamaguchi S, et al. Cryptococcus arboriformis Sp. Nov., a novel anamorphic basidiomycetous yeast species isolated from a patient's urine. Microbiol Immunol. 2007; 51:543–545. PMID: 17579264.
11. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005; 25:107–131. PMID: 15796137.

12. Chang TI, Kim HW, Park JT, Lee DH, Lee JH, Yoo TH, et al. Early catheter removal improves patient survival in peritoneal dialysis patients with fungal peritonitis: results of ninety-four episodes of fungal peritonitis at a single center. Perit Dial Int. 2011; 31:60–66. PMID: 20505136.

13. Keane WF, Bailie GR, Boeschoten E, Gokal R, Golper TA, Holmes CJ, et al. Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit Dial Int. 2000; 20:396–411. PMID: 11007371.

14. Fera MT, La Camera E, De Sarro A. New triazoles and echinocandins: mode of action, in vitro activity and mechanisms of resistance. Expert Rev Anti Infect Ther. 2009; 7:981–998. PMID: 19803707.
15. Chmielewski M, Lichodziejewska-Niemierko M, Liberek T, Naumiuk L, Samet A, Rutkowski B. Ciprofloxacin as adjunctive agent in treating cryptococcal peritonitis. Perit Dial Int. 2004; 24:487–488. PMID: 15490991.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download