Abstract
In up to 40% of systemic mastocytosis (SM) cases, an associated clonal hematological non-mast cell lineage disease such as AML is diagnosed before, simultaneously with, or after the diagnosis of SM. A 40-yr-old man was diagnosed with AML with t(8;21)(q22;q22). Mast cells were not noted at diagnosis, but appeared as immature forms at relapse. After allogeneic hematopoietic stem cell transplantation (HSCT), leukemic myeloblasts were not observed; however, neoplastic metachromatic blasts strikingly proliferated during the state of bone marrow aplasia, and finally, aleukemic mast cell leukemia developed. As the disease progressed, we observed serial morphologic changes from immature mast cells with myeloblasts to only metachromatic blasts and atypical mast cells as mast cell leukemia; FISH analysis showed that the neoplastic mast cells originated from the same clone as the leukemic myeloblasts of AML.
Mastocytosis is defined as a clonal, neoplastic proliferation of mast cells with accumulation in one or multiple organs [1]. The clinical presentation is heterogeneous, ranging from skin-limited disease (cutaneous mastocytosis) that spontaneously regresses to a highly aggressive variant with extracutaneous involvement (systemic mastocytosis, SM) associated with multiorgan failure and short survival [2]. SM with associated clonal hematological non-mast cell lineage disease, which is characterized by the presence of the KIT mutation, is the second most common type of SM, representing 40% of cases [2]. AML with RUNX1-RUNX1T1, frequently seen in cases of SM with AML, also occasionally has the KIT-activating mutational codon D816 [3, 4]. Both SM and RUNX1-RUNX1T1-positive AML with the KIT mutation at codon D816 have been reported to be refractory to chemotherapy [3, 4]. However, the relationship between the 2 coexisting clonal disorders remains unknown. Here, we describe the clinical and laboratory features of neoplastic mast cell proliferation in a patient with RUNX1-RUNX1T1 and KIT-D816 mutation-positive AML terminating as aleukemic mast cell leukemia after allogeneic hematopoietic stem cell transplantation (HSCT). To the best of our knowledge, no report has described the sequential morphologic alterations of neoplastic mast cells progressing to mast cell leukemia.
A 40-yr-old Korean man presented with dyspnea on exertion. In the peripheral blood (PB), the white blood cell (WBC) count was 5×109/L and consisted of 68% myeloblasts and no mast cells. The bone marrow (BM) was hypercellular and included 90.4% typical myeloblasts and no mast cells. The karyotype was 45,X,-Y,t(8;21)(q22;q22). He was diagnosed with AML with t(8;21)(q22;q22) according to the WHO criteria [1]. He received 1 cycle of combination chemotherapy with idarubicin and cytosine arabinoside and 2 cycles of high-dose chemotherapy with cytosine arabinoside within a period of 5 months. At the end of the first cycle of chemotherapy, complete remission of AML was achieved. The sequential high-dose chemotherapy cycle was complicated with intracranial hemorrhage and severe sepsis, but the patient recovered without any sequelae.
Eight months after the diagnosis, the patient relapsed with clonal evolution. The karyotype was 45,X,-Y,t(8;21)(q22;q22), del(17)(p11.2). In spite of salvage chemotherapy, hematological remission was not achieved. PB showed pancytopenia with WBC count of 1.9×109/L, hemoglobin of 10.6 g/dL, and platelet count of 28×109/L. The differential count showed 16% myeloblasts and 1% circulating immature mast cells. BM aspirate smear showed 31.2% myeloblasts and 5.8% immature mast cells. The myeloblasts showed medium-sized, oval- to irregular-shaped nuclei, prominent nucleoli, and scant basophilic cytoplasm (Fig. 1A-1, A-2). Flow cytometric immunophenotyping showed that the leukemic myeloblasts expressed CD34, HLA-DR, CD13, CD33, CD117, CD65, CD15, and CD56, but lacked CD2 (Fig. 2A). BM trephine biopsy revealed persistent leukemic myeloblasts and interstitial infiltration of mast cells. Immunohistochemically, these mast cells were positive for tryptase and CD25 (Fig. 1A-3). Reverse transcriptase (RT)-PCR of the BM aspirate showed RUNX1-RUNX1T1 fusion transcript and KIT mutation at codon 816. The patient was diagnosed with SM-AML with t(8;21)(q22;q22).
He received HSCT from a HLA-haploidentical donor. The conditioning regimen included busulfan and fludarabine. Twenty-nine days after HSCT, the patient's PB WBC count was 0.8×109/L with 17% metachromatic blasts. BM aspirates also contained metachromatic blasts (62.4%) showing a high nuclear-to-cytoplasmic (N/C) ratio, immature nuclear chromatin, inconspicuous nucleoli, and several metachromatic granules (Fig. 1B-1, B-2). Metachromatic blasts infiltrated as interstitial type and focally aggregated in a BM biopsy specimen (Fig. 1B-3).
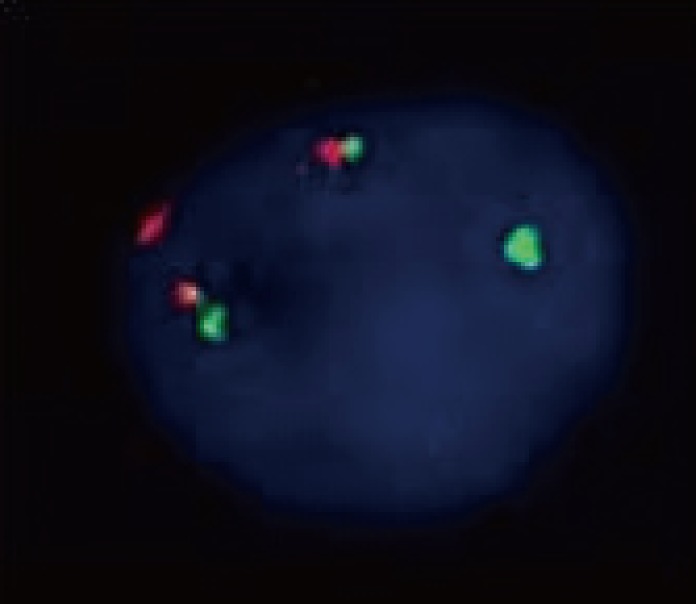
On day 49 after HSCT, pancytopenia worsened, with WBC count of 0.2×109/L, hemoglobin of 8.7 g/dL, and platelet count of 11×109/L; however, myeloblasts or mast cells were not seen in the PB. Instead of them, atypical mast cells (type II) constituted 69.2% of all nucleated cells in a BM aspirate smear (Fig. 1C-1, C-2) [5] and were compactly infiltrated in a BM biopsy sample (Fig. 1C-3). Leukemic myeloblasts were not seen in the BM aspirate smear. On flow cytometric analysis, these atypical mast cells expressed CD13, CD33, CD117, CD25, and CD56, but lacked CD34 and HLA-DR, which were expressed strongly at relapse (Fig. 2B). The karyotype was 45,X,-Y,t(8;21)(q22;q22),del (17)(p11.2)[5]//46,XX[15]. FISH using a Vysis RUNX1/RUNX1T1 Dual Color, Dual Fusion Translocation Probe (Abbott Laboratories, Abbott Park, IL, USA) revealed RUNX1-RUNX1T1 rearrangement as nuc ish(RUNX1T1, RUNX1)x3(RUNX1T1 con RUNX1x2)[58/100] (Fig. 3). The atypical mast cells also showed a RUNX1-RUNX1T1 fusion transcript and a KIT 816 mutation on RT-PCR. At this time, the patient was diagnosed with aleukemic mast cell leukemia.
He died 4 months after HSCT because of a new intracranial hemorrhage.
This case was a diagnostic challenge because it appeared to be a case of AML with t(8;21)(q22;q22) without evidence of mastocytosis at the initial diagnosis but terminated as mast cell leukemia after HSCT. In cases of SM-AML with t(8;21)(q22;q22) in the literature, diagnoses of SM were made either before or at the same time as the diagnosis of AML or after chemotherapy [6-10]. Our patient was diagnosed with SM-AML at the time of relapse after chemotherapy; however, we cannot exclude the possibility that SM was present at the initial presentation but was masked by excessive blast accumulation.
In its morphologic aspect, the neoplastic mast cell has been classified into 3 subtypes: metachromatic blast and atypical mast cell types I and II [5]. Metachromatic blasts demonstrate blast-like nuclear morphology with several metachromatic granules. Atypical mast cells type I show elongated cytoplasmic projections, oval eccentric nuclei, and hypogranulation, whereas atypical mast cells type II typically have bilobed or polylobed nuclei. In aggressive SM cases and in mast cell leukemia, many neoplastic mast cells exhibit more prominent cellular atypia such as metachromatic blasts or atypical mast cells type II. In our case, we found extremely enhanced proliferation of neoplastic mast cells and morphologically metachromatic blasts without leukemic myeloblasts on day 29 after HSCT. These metachromatic blasts were medium-sized cells with fine nuclear chromatin and pale basophilic cytoplasm containing a variable number of metachromatic granules. On day 49 after HSCT, these cells had transformed to atypical mast cells type II with bilobed nuclei or multiple nuclei, more condensed nuclear chromatin, pale cytoplasm, and coarse metachromatic granules.
KIT mutations are associated with enhanced proliferation of mast cells and are frequently detectable in a group of patients with core-factor binding leukemias, such as AML with inv(16) or t(8;21)(q22;q22). In such cases, D816V KIT is the most common mutation found [11]. We could clearly detect the KIT-D816 mutation, not only from the BM aspirate containing 31.2% of leukemic myeloblasts and 5.8% of immature mast cells but also from BM aspirates with 62.4% of metachromatic blasts (day 29 post-HSCT), 69.2% of atypical mast cells (day 49 post-HSCT), and no leukemic myeloblasts.
Published data regarding SM-AML have shown different aspects of the clonal origins of mast cells. The presence of the KIT mutation at codon D816 or the RUNX1-RUNX1T1 rearrangement in mast cells but not in leukemic myeloblasts in the same patient shows that mast cells and leukemic myeloblasts are possibly of different clonal origins or 2 separately developed subclones [6, 7]. However, in other cases, a common clonal origin of mast cells and leukemic myeloblasts has been suggested according to FISH analysis with a probe specific for RUNX1-RUNX1T1 on sorted mast cells [9, 10, 12]. We proved a D816 KIT mutation by using RT-PCR and RUNX1-RUNX1T1 expression by using FISH in both cell lineages. Thus, we provide evidence that neoplastic mast cells and myeloid leukemic blasts are likely to develop from common hematopoietic progenitors. On the other hand, an article regarding the origin of mast cells after allogeneic HSCT reported that the proliferated mast cells had originated from the donor [13], but in our case, a male patient received a transplant from a female donor, and so we could easily exclude that possibility by cytogenetic analysis.
Clinically significant bleeding can be caused by a high level of heparin after mast cell degranulation [14]. Thus, we conclude that a bleeding diathesis resulting from excessive atypical mast cells is strongly associated with intracranial hemorrhage, the main cause of this patient's death. Several studies about SM-AML reported that after successful HSCT, the neoplastic mast cells gradually decline with time [8-10], probably because of a graft-versus-mast cell effect or gradual apoptosis [9]. Because continued mastocytosis can elevate the risk of bleeding, it may be necessary to consider aggressive interventions like donor lymphocyte infusion [15]. Thus, in cases of SM-AML, whether HSCT is successful or not, a careful morphologic examination of the mast cells is necessary.
In conclusion, we report a case of SM-AML that terminated as aleukemic mast cell leukemia after HSCT, and describe the serial morphologic changes in neoplastic mast cells.
References
1. Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, et al. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. Mastocytosis. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: IARC Press;p. 54–63.
2. Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009; 113:5727–5736. PMID: 19363219.

3. Valent P, Akin C, Sperr WR, Mayerhofer M, Födinger M, Fritsche-Polanz R, et al. Mastocytosis: pathology, genetics, and current options for therapy. Leuk Lymphoma. 2005; 46:35–48. PMID: 15621779.

4. Schnittger S, Kohl TM, Haferlach T, Kern W, Hiddemann W, Spiekermann K, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006; 107:1791–1799. PMID: 16254134.

5. Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001; 25:603–625. PMID: 11377686.

6. Sperr WR, Walchshofer S, Horny HP, Födinger M, Simonitsch I, Fritsche-Polanz R, et al. Systemic mastocytosis associated with acute myeloid leukaemia: report of two cases and detection of the c-kit mutation Asp-816 to Val. Br J Haematol. 1998; 103:740–749. PMID: 9858225.

7. Escribano L, Garca-Montero A, Núñez-Lopez R, López-Jiménez J, Almeida J, Prados A, et al. Systemic mastocytosis associated with acute myeloid leukemia: case report and implications for disease pathogenesis. J Allergy Clin Immunol. 2004; 114:28–33. PMID: 15241340.

8. Bernd HW, Sotlar K, Lorenzen J, Osieka R, Fabry U, Valent P, et al. Acute myeloid leukaemia with t(8;21) associated with "occult" mastocytosis. Report of an unusual case and review of the literature. J Clin Pathol. 2004; 57:324–328. PMID: 14990611.

9. Pullarkat V, Bedell V, Kim Y, Bhatia R, Nakamura R, Forman S, et al. Neoplastic mast cells in systemic mastocytosis associated with t(8;21) acute myeloid leukemia are derived from the leukemic clone. Leuk Res. 2007; 31:261–265. PMID: 16876862.

10. Nagai S, Ichikawa M, Takahashi T, Sato H, Yokota H, Oshima K, et al. The origin of neoplastic mast cells in systemic mastocytosis with AML1/ETO-positive acute myeloid leukemia. Exp Hematol. 2007; 35:1747–1752. PMID: 17976525.

11. Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006; 24:3904–3911. PMID: 16921041.
12. Sperr WR, Drach J, Hauswirth AW, Ackermann J, Mitterbauer M, Mitterbauer G, et al. Myelomastocytic leukemia: evidence for the origin of mast cells from the leukemic clone and eradication by allogeneic stem cell transplantation. Clin Cancer Res. 2005; 11:6787–6792. PMID: 16203765.

13. Chen TY, Chen JS, Huang WT, Su WC, Tsao CJ. Rapid engraftment of mast cells of donor origin in a case of acute myeloid leukemia with mast cell leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003; 32:111–114. PMID: 12815487.

14. Parker RI. Hematologic aspects of systemic mastocytosis. Hematol Oncol Clin North Am. 2000; 14:557–568. PMID: 10909040.

15. Spyridonidis A, Thomas AK, Bertz H, Zeiser R, Schmitt-Graff A, Lindemann A, et al. Evidence for a graft-versus-mast-cell effect after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004; 34:515–519. PMID: 15273711.

Fig. 1
Serial findings in peripheral blood (PB), bone marrow (BM) aspirate (Wright stain, ×1,000), and a BM biopsy specimen (tryptase immunohistochemical stain, ×40) at relapse (A), day 29 post hematopoietic stem cell transplantation (HSCT) (B), and day 49 post-HSCT (C). (A-1) A leukemic myeloblast (left) and an immature mast cell with inconspicuous nucleoli (right) in PB. (A-2) An immature mast cell (left), and a leukemic myeloblast (right) in BM aspirate. (A-3) Hypercellular marrow with interstitial infiltration of mast cells in a BM biopsy specimen. (B-1) A metachromatic blast with a high nuclear-to-cytoplasmic (N/C) ratio, fine nuclear chromatin, inconspicuous nucleoli, hypogranular cytoplasm, and variable number of metachromatic granules in PB. (B-2) Metachromatic blasts in BM aspirate. (B-3) Hypercellular marrow with interstitial and focally aggregated infiltration of metachromatic blasts in a BM biopsy specimen. (C-1) and (C-2) Atypical mast cells with bilobed or polylobed nuclei, high or low N/C ratio, relatively dense nuclear chromatin, no nucleoli, pale cytoplasm, and abundant metachromatic granules in BM aspirate. (C-3) Hypercellular marrow with packed infiltration of atypical mast cells in a BM biopsy specimen.
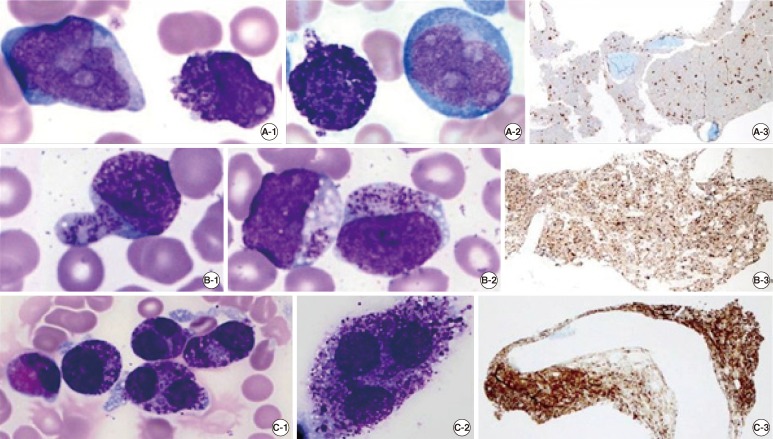
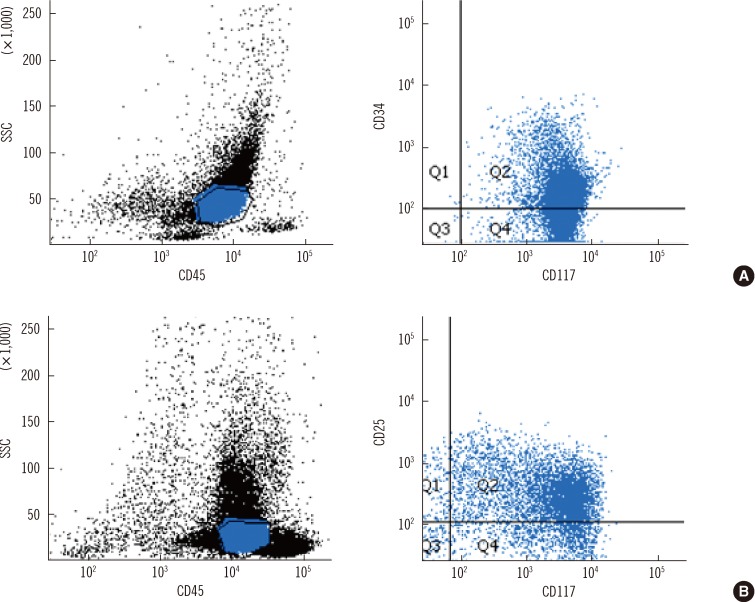
Fig. 2
Immunophenotypic findings of bone marrow aspirate at relapse (A) and on day 49 post-HSCT (B). (A) Leukemic myeloblasts with CD45 intermediate expression show the positivity of immature myeloid markers (CD34 and CD117) at relapse. (B) Atypical mast cells with CD45 intermediate to bright expression show the positivity of neoplastic mast cell markers (CD117 and CD25) on day 49 post-HSCT.
Abbreviation: SSC, side scatter.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download