Abstract
Purpose
The purpose of this study is to identify risk factors for severe thyroid-associated orbitopathy (TAO) and compressive optic neuropathy in Korean patients.
Methods
This study was a retrospective comparative case series. All TAO patients who were first seen at our institution between 2005 and 2009 and who had follow-up periods of at least 6 months were included. Patients were divided into mild or moderate and severe TAO groups. Cases were also segregated based on the presence or absence of optic neuropathy. Demographics, smoking status, comorbidities, thyroid hormonal status, thyroid autoantibody levels, and clinical presentations were assessed.
Results
A total of 99 patients (83 with mild to moderate courses and 16 with severe courses; 90 without optic neuropathy and 9 with optic neuropathy) were included in this study. On multiple logistic regression analysis, smoking status was a predictive risk factor for a severe course of TAO and the development of optic neuropathy (odds ratios = 6.57 and 10.00, respectively). Other factors such as age, gender, free T4 level, thyroid binding-inhibiting immunoglobulin, and a history of diabetes were not predictive of severe TAO or optic neuropathy.
Conclusions
Although various factors may influence the severity of TAO and the development of optic neuropathy, this study showed that smoking was a risk factor for severe TAO and the development of optic neuropathy. Therefore, it is important for patients with Graves' disease to refrain from smoking. Frequent and careful observation should also be performed in current smokers, as TAO patients who smoke are susceptible to a severe course and/or optic neuropathy.
Thyroid-associated orbitopathy (TAO) is an autoimmune disorder that poses a major clinical and therapeutic challenge. The clinical manifestations range from mild findings such as tearing, photophobia, and conjunctival injection, to more significant findings including proptosis, diplopia, exposure keratopathy, and vision loss from compressive optic neuropathy. In the majority of patients, ocular involvement is not severe and is self-limiting, but 3 to 5% of patients have severe and progressive ocular manifestations [1]. Severe TAO represents a complex therapeutic challenge and approximately one-third of patients are not satisfied with their therapeutic outcome at the end of follow-up [2]. The onset and progression of TAO are influenced by several factors that are potentially modifiable such as cigarette smoking, thyroid dysfunction, and choice of treatment modalities for hyperthyroidism [3-9]. The objective of this study was to identify Korean patients at increased risk of a severe course of TAO and compressive optic neuropathy. In this study, we used multivariate logistic regression analysis to compare possible risk factors and various clinical variables between patients with mild to moderate courses and severe courses of TAO and also between patients with and without optic neuropathy.
Enrollment was restricted to patients who were initially seen for TAO at our institution between 2005 and 2009 within 6 months after initial onset and who were followed-up for at least 6 months. Ninety-nine patients were eligible for analysis. This study complied with the policies of the local Institutional Review Board.
The diagnosis of TAO was based on the presence of typical clinical features of the disease, including eyelid retraction, proptosis, impaired motility, an increase in intraocular pressure on upward gaze, one or more enlarged extraocular muscles, and increased intraorbital fat on computed tomography scans. In this study, a mild course of TAO was defined as proptosis less than 21 mm with no or only intermittent diplopia without optic nerve involvement. Moderate TAO was defined as proptosis between 21 mm and 23 mm with intermittent diplopia and no optic nerve involvement [1]. Patients were judged to have a severe course of TAO if they had, in the worse eye, either motility impairment causing constant diplopia within 30 degrees by the binocular single visual field test and the Hess screen (Fig. 1); or proptosis greater than 23 mm or with a difference between eyes of more than 5 mm by Hertel exophthalmometry, causing serious exposure keratopathy (Fig. 2); or compressive optic neuropathy (Fig. 3). Optic neuropathy was diagnosed if more than two of the following symptoms and signs were present: acutely reduced vision, abnormal color contrast, relative afferent pupillary defect (RAPD), visual field defect by Humphrey visual field testing, disc edema and swelling, or apical crowding of the extraocular muscles on a computed tomography scan.
Details on patient demographics including age, sex, prior history of hyperthyroidism, onset of TAO associated with thyroid disease, comorbidities such as diabetes, hypertension, and myasthenia gravis, and smoking status were recorded.
All patients were examined at least twice (every 3 months) after presentation. At each visit, the serum free T4 level (nor mal 0.73-1.95 ng/dL) and thyroid binding-inhibiting immunoglobulin (TBII, positive when more than 10 U/L) level were measured. Eye manifestations were assessed using the following parameters: visual acuity, intraocular pressure, presence of lid retraction, and proptosis as measured by Hertel exophthalmometry. Ocular horizontal and vertical ductions were judged on a subjective scale of 0 to -4. The degree of diplopia was assessed objectively by the binocular single vision test and subjectively by the Gorman score: grade 0, no diplopia; 1, intermittent; 2, inconstant (gaze evoked); and 3, constant diplopia in primary gaze or while reading [10]. Disease activity was evaluated using the 7-point modified formulation of the clinical activity score by Mourits et al. [11,12]. In patients with reduced visual acuity, the Ishihara color test, the light pupil reflex test, fundus examination, the Humphrey visual field test, and an orbital computed tomography scan were performed to diagnose optic neuropathy.
In patients with compressive optic neuropathy, we used intravenous methylprednisolone (1 g per day for 3 days) and an oral prednisolone taper thereafter. If vision did not return to normal or abnormal Ishihara test results, an RAPD, or visual field defects persisted, then surgical decompression was advised. In patients with a severe active stage (clinical activity score ≥3) without evidence of optic neuropathy, oral prednisolone was given for 3 months in decreasing doses: 60 mg for the first 2 weeks, 40 mg for 2 weeks, 30 mg for 4 weeks, and 20 mg for 4 weeks. Although intravenous glucocorticoids are known to be more effective than oral steroids [13], oral steroids were chosen in most patients in this study because of difficulties in attending frequent clinic visits. In patients with a mild to moderate course, prednisolone was started at a lower dose (40 mg for 2 weeks) if they were in an active stage and reported a recent aggravation of ocular symptoms and signs.
Retrobulbar radiotherapy was not administered in this series. If activity reappeared, prednisolone was again given in the same manner. Rehabilitative surgery was only performed in patients with inactive TAO (clinical activity score <3) for at least 6 months.
Demographic, clinical, and biochemical variables between patients with mild to moderate courses of TAO and severe courses of TAO, and between patients with and without compressive optic neuropathy, were compared using independent t-tests for continuous variables and chi-square or Fisher's exact tests for categorical data.
Multivariate logistic regression was performed to investigate the influence of age, gender, free T4 level (more than 3 ng/dL), positive TBII level (more than 10 U/L), diabetes, and smoking status on both the severity of TAO and the development of optic neuropathy. Odds ratios with 95% confidence intervals were calculated. All statistical analyses were performed by a computer using SPSS ver. 12.0 (SPSS, Inc., Chicago, IL, USA).
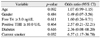
Out of a total of 99 patients who were eligible for analysis, 83 were diagnosed with mild to moderate TAO and 16 were diagnosed with severe TAO. Demographic, clinical, and biochemical features were compared between patients with mild to moderate TAO and severe TAO (Table 1). There were older patients among those with severe courses than in the group with mild to moderate courses (t-test, p = 0.007). There were more male patients in patients with a severe course (43.8%) compared to those with a mild to moderate course (20.5%); however, this difference was not statistically significant (chi-square test, p = 0.060). There was no statistically significant difference between the two groups with regard to length of the follow-up period (t-test, p = 0.967). The initial free T4 levels were high (above 3 ng/dL) in 30.0% of the mild to moderate group and in 21.4% of the severe group. The ratio of patients with positive TBII was similarly high in both groups; 86.7% in the mild to moderate group and 90.0% in the severe group. However, there was no statistical significance with respect to the number of patients with a high initial free T4 level (>3.0 ng/dL) and positive TBII (>10 U/L) between the two groups (chi-square test, p = 0.748; chi-square test, p = 1.000, respectively). More patients with a severe course had a history of diabetes (chi-square test, p = 0.029). More patients with a severe course were smokers compared to those with a mild to moderate course (chi-square test, p = 0.021). More patients with a severe course had a Gorman score of 2 or 3 (chi-square test, p = 0.036) and a higher clinical activity score (chi-square test, p < 0.001; t-test, p = 0.005). There was no statistically significant difference in restrictive myopathy between the two groups (chi-square test, p = 0.444).
On multiple logistic regression analysis, smoking behavior was found to be a risk factor for severe TAO with an odds ratio of 6.57 (Table 2). A history of diabetes and a positive TBII level also revealed odds ratios of 2.36 and 2.57, respectively; however, they were not statistically significant.
When demographic, clinical, and biochemical variables were compared in patients with and without optic neuropathy, patients with optic neuropathy were older on average (t-test, p = 0.001) (Table 3). A higher ratio of the male gender was found in the group with optic neuropathy, but this difference was not statistically significant (chi-square test, p = 0.214). There was no significant difference between the two groups regarding the percentage of patients with a high initial free T4 level above 3.0 ng/dL and positive TBII (chi square test, p = 1.000). More patients with optic neuropathy had a history of diabetes and hypertension, but those differences were not statistically significant (chi-square test, p = 0.064). In addition, patients with optic neuropathy exhibited more exophthalmos (t-test, p = 0.018), a higher Gorman score of 2 or 3 (chi-square test, p = 0.036), and a higher clinical activity score (t-test, p < 0.001) than those without optic neuropathy.
Multiple logistic regression analysis revealed that smoking influenced the development of optic neuropathy, with an odds ratio of 10.00 (Table 4). A history of diabetes, free T4 level ≥3.0 ng/dL, and positive TBII had an odds ratio of 3.90, 2.31, and 1.47, respectively, but they were not statistically significant.
The clinical diagnosis of TAO is relatively easy; however, clinical decisions regarding steroid treatment, irradiation, or surgical rehabilitation are often difficult. The natural course of TAO is benign in most patients and improvement is often seen with only conservative treatment. However, some patients develop progressive severe ophthalmopathy and require prolonged anti-inflammatory therapy. Clinical decisions would be easier if the course of TAO could be predicted.
Several risk factors including old age, male gender, smoking, and abnormal thyroid function have been identified in several reports as possible predisposing factors for severe TAO. A positive relationship between age and ophthalmopathy index has been identified; also, males were found to have an average ophthalmopathy index 41% greater than that of females after correcting for age [14]. Several cross-sectional and cohort studies have shown that smoking is an independent risk factor for severe ophthalmopathy [6,15-19]. One review journal provided strong evidence for a causal association between smoking and the development of TAO, with a risk ratio/odds ratio >2 in most studies, although the quality of the studies varied [17]. Thyroid status was influential in determining the clinical course of ophthalmopathy in several reports [5,7,8,20-22]. In the past, euthyroid Graves' disease was reported to be a predictor of poor prognosis in patients who receive orbital irradiation [22]. In one recent report, patients in a normal thyroid function group were significantly older, more likely to be male, and had less severe ophthalmopathy than did patients in an abnormal thyroid function group [5].
In our analysis, patients with severe TAO were older, more likely to have diabetes, and were more likely to be smokers than patients with mild to moderate courses of TAO. Thyroid status as assessed by the initial free T4 level, TBII, and TAO duration were not associated with severe TAO. Turning to the clinical features, patients with severe TAO showed a higher degree of exophthalmos, more severe diplopia, more severe restrictive myopathy, and higher clinical activity scores. However, in the multiple logistic regression analysis only smoking was predictive of severe TAO.
TAO in patients with optic neuropathy is almost always active and requires urgent treatment. It is essential that the correct diagnosis be made. In the European Group on Graves' Orbitopathy survey, there was no defined gold standard for the diagnosis of optic neuropathy [23,24]. Three criteria including radiologic evidence of optic neuropathy (apical crowding), reduced visual acuity, and impaired color vision were highly sensitive and specific in making a clinical diagnosis of optic neuropathy. In patients with a crowded apex on scanning, clinically diagnosed optic neuropathy was present in 88% of cases [23,24]. To better define this condition, Dayan and Dayan [23] recommended a diagnostic algorithm for optic neuropathy that included radiologic evidence of optic nerve compression. Although this criteria is not always necessary in the definition of optic neuropathy [25], we believe radiologic assessment showing apical crowding or fat prolapse through the superior orbital fissure can confirm the presence of optic neuropathy and exclude alternative causes. Optic neuropathy has been found to be correlated with both total extraocular muscle volume and a limitation of ocular motility, which suggests that optic nerve involvement is more likely to occur in association with noncompliant fibrotic muscle versus more supple muscle of the same total volume [26]. Male gender, old age, diabetes, and heavy smoking were reported as other risk factors [9].
In our series, age, smoking, exophthalmos, myopathy, and CAS were found to be related to the development of optic neuropathy. The absence of severe proptosis in dysthyroid optic neuropathy patients has previously been reported [27]. A lack of proptosis could increase orbital pressure, precipitating optic neuropathy in some cases [28]. However, in our study, patients with optic neuropathy exhibited greater exophthalmometry than patients without optic neuropathy (p = 0.018). This could result from the inclusion of patients with a mild to moderate course into the group without optic neuropathy. If the comparison was confined to patients with a severe course, the results could be similar to the previous reports. Among those patients, smoking was the only predictive factor of optic neuropathy, with an odds ratio of 10.00 (p = 0.033).
A number of experimental and clinical studies support the idea that TSH-receptor autoantibodies, such as the TBII or thyroid-stimulating antibody, are involved in the pathophysiology of TAO [29-31]. In one study of Asian patients, the level of thyroid-stimulating autoantibodies and the absence of anti-thyroid peroxidase antibodies were independent risk factors for TAO, whereas no correlations between TBII or anti-thyroglobulin antibody levels and ophthalmopathy were found [30]. In our study, TBII levels were frequently positive (more than 10 U/L) in both the mild to moderate and severe groups (86.7% and 90.0%, respectively), but this difference was not statistically significant.
We have confirmed that smoking is the strongest risk factor for development of a severe course of TAO and optic neuropathy in Korean patients, as observed in previous studies [6,15-19]. In an in vitro model of TAO, cigarette smoke extract increased glycosaminoglycan production and adipogenesis in orbital fibroblasts [32]. Thus, it is important for patients with Graves' disease to refrain from smoking. In addition, frequent and careful observation should be performed in current smokers, as patients who smoke are susceptible to a more severe course of TAO and/or optic neuropathy.
Figures and Tables
Fig. 1
The binocular single visual field test (A) and Hess screen (B) of a patient with severe restrictive myopathy causing constant diplopia within 30 degrees.

Fig. 2
Photograph (A), axial (B), and coronal computed tomography scans (C) of a patient with unilateral proptosis, with a difference of more than 5 mm by Hertel exophthalmometry, causing exposure keratopathy.
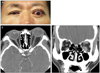
Fig. 3
Photograph (A), axial (B), and coronal computed tomography scans (C) of a patient with bilateral compressive optic neuropathy.
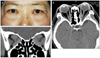
Table 1
Comparison of demographic, clinical, and biochemical features between patients with mild to moderate courses and severe courses of thyroid-associated orbitopathy

Table 2
Variables predictive of the severity of thyroidassociated orbitopathy on multiple logistic regression
analysis
References
1. Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev. 2000. 21:168–199.
2. Bartley GB, Fatourechi V, Kadrmas EF, et al. Long-term follow-up of Graves ophthalmopathy in an incidence cohort. Ophthalmology. 1996. 103:958–962.
3. Bartalena L, Marcocci C, Pinchera A. Graves' ophthalmopathy: a preventable disease? Eur J Endocrinol. 2002. 146:457–461.
4. Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002. 12:855–860.
5. Kim JM, LaBree L, Levin L, Feldon SE. The relation of Graves' ophthalmopathy to circulating thyroid hormone status. Br J Ophthalmol. 2004. 88:72–74.
6. Prummel MF, Wiersinga WM. Smoking and risk of Graves' disease. JAMA. 1993. 269:479–482.
7. Prummel MF, Wiersinga WM, Mourits MP, et al. Effect of abnormal thyroid function on the severity of Graves' ophthalmopathy. Arch Intern Med. 1990. 150:1098–1101.
8. Tallstedt L, Lundell G, Torring O, et al. The Thyroid Study Group. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. N Engl J Med. 1992. 326:1733–1738.
9. Wiersinga WM. Management of Graves' ophthalmopathy. Nat Clin Pract Endocrinol Metab. 2007. 3:396–404.
10. Bahn RS, Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid-associated ophthalmopathy. Endocrinol Metab Clin North Am. 1987. 16:391–407.
11. Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf). 1997. 47:9–14.
12. Classification of eye changes of Graves' disease. Thyroid. 1992. 2:235–236.
13. Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves' orbitopathy. J Clin Endocrinol Metab. 2005. 90:5234–5240.
14. Perros P, Crombie AL, Matthews JN, Kendall-Taylor P. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf). 1993. 38:367–372.
15. Tallstedt L, Lundell G, Taube A. Graves' ophthalmopathy and tobacco smoking. Acta Endocrinol (Copenh). 1993. 129:147–150.
16. Winsa B, Mandahl A, Karlsson FA. Graves' disease, endocrine ophthalmopathy and smoking. Acta Endocrinol (Copenh). 1993. 128:156–160.
17. Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye (Lond). 2007. 21:1135–1145.
18. Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol (Oxf). 1996. 45:477–481.
19. Bartalena L, Marcocci C, Tanda ML, et al. Cigarette smoking and treatment outcomes in Graves ophthalmopathy. Ann Intern Med. 1998. 129:632–635.
20. Solomon DH, Chopra IJ, Chopra U, Smith FJ. Identification of subgroups of euthyroid Graves' ophthalmopathy. N Engl J Med. 1977. 296:181–186.
21. Bhatnagar A, Tsirbas A, Douglas RS, et al. Graves' orbitopathy. Ophthalmology. 2007. 114:392.
22. Petersen IA, Kriss JP, McDougall IR, Donaldson SS. Prognostic factors in the radiotherapy of Graves' ophthalmopathy. Int J Radiat Oncol Biol Phys. 1990. 19:259–264.
23. Dayan CM, Dayan MR. Dysthyroid optic neuropathy: a clinical diagnosis or a definable entity? Br J Ophthalmol. 2007. 91:409–410.
24. Bartalena L, Baldeschi L, Dickinson AJ, et al. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid. 2008. 18:333–346.
25. Soares-Welch CV, Fatourechi V, Bartley GB, et al. Optic neuropathy of Graves disease: results of transantral orbital decompression and long-term follow-up in 215 patients. Am J Ophthalmol. 2003. 136:433–441.
26. Feldon SE, Muramatsu S, Weiner JM. Clinical classification of Graves' ophthalmopathy. Identification of risk factors for optic neuropathy. Arch Ophthalmol. 1984. 102:1469–1472.
27. Mourits MP, Lombardo SH, van der Sluijs FA, et al. Reliability of exophthalmos measurement and the exophthalmometry value distribution in a healthy Dutch population and in Graves' patients. An exploratory study. Orbit. 2004. 23:161–168.
28. McKeag D, Lane C, Lazarus JH, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves' Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007. 91:455–458.
29. Burch HB, Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993. 14:747–793.
30. Khoo DH, Ho SC, Seah LL, et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves' disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999. 9:1175–1180.
31. Gerding MN, vanderMeer JW, Broenink M, et al. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf). 2000. 52:267–271.
32. Cawood TJ, Moriarty P, O'Farrelly C, O'Shea D. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J Clin Endocrinol Metab. 2007. 92:59–64.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download