Abstract
Purpose
To evaluate therapeutic effects and usefulness of a combination treatment of intravitreal injection of triamcinolone acetonide (IVTA) and panretinal photocoagulation (PRP) in patients with clinically significant macular edema secondary to proliferative diabetic retinopathy (PDR).
Methods
Visual acuity test, fundoscopy, fluorescein angiography, and optical coherence tomography (OCT) were taken in 20 patients (20 eyes) of macular edema and PDR. A combination of intravitreal injection of triamcinolone acetonide and PRP was performed in 10 patients (10 eyes) and a combination of focal or grid laser photocoaqulation and PRP in the remaining 10 eyes. The postoperative outcomes were compared between the two combination treatments by best corrected visual acuity (BCVA), tonometry, fluorescein angiography, and OCT at 2 weeks, 1, 2, and 3 months.
Results
Average BCVA (log MAR) significantly improved from preoperative 0.56±0.20 to 0.43±0.08 at 1 month (P=0.042) and it was maintained until 3 months after a combination of IVTA and PRP in 10 eyes (P=0.007). The thickness of fovea decreased from average 433.3±114.9 µm to average 279.5±34.1 µm at 2 weeks after combined treatment of IVTA and PRP (P=0.005), which was significantly maintained until 3 months, but there was a transient visual disturbance and no significant difference in thickness of the fovea before and after treatment in the groups with PRP and focal or grid laser photocoagulation.
Conclusions
A combination of IVTA and PRP might be an effective treatment modality in the treatment of macular edema and PDR and prevent the subsequent PRP-induced macular edema result in visual dysfunction. In combination with PRP, IVTA might be more effective than focal or grid laser photocoagulation and PRP for reducing diabetic macular edema and preventing aggravation of macular edema without transient visual disturbance in patients requiring immediate PRP.
Diabetic macular edema is the leading cause of decrease in visual acuity and may frequently lead to irreversible changes in visual acuity. Focal or grid laser photocoagulation has been performed for diabetic macular edema, but it has been known that laser photocoagulation requires a considerable time for recovery of macular edema and moderate visual loss can be prevented with little improvement of visual acuity only in 50% of patients.1-4 Panretinal photocoagulation (PRP) is an effective method to prevent neovascularization and progression of proliferative diabetic retinopathy (PDR), but aggravation of macular edema with decrease in visual acuity may occur in diabetic retinopathy accompanied by macular edema after PRP.5-9 When PRP is performed after focal or grid laser therapy for macular edema, moderate visual loss can be reduced, but the timing of PRP may be delayed because this procedure should be performed after sufficient stabilization of macular edema.1,5,8
Intravitreal injection of triamcinolone acetonide (IVTA) is effective in the elimination of macular edema and improvement of visual acuity, especially in diabetic macular edema unresponsive to laser therapy, so it has recently been in wide use.10,11 It has been suggested that alleviation of macular edema by triamcinolone acetonide (TA) may prevent aggravation of macular edema secondary to PRP.
This study was designed to evaluate therapeutic effects and usefulness of a combination of a PRP combined with IVTA and a PRP combined with focal or grid laser photocoagulation in order to maximize the effect of laser photocoagulation and reduce macular edema, which may be aggravated after PRP, in patients with both clinically significant macular edema (CSME) and PDR requiring immediate PRP.
Anterior segment examination, best-corrected visual acuity (BCVA), tonometry, and fundus examination were taken in patients with CSME secondary to diabetic retinopathy. The severity level of diabetic retinopathy was determined as defined in a diabetic retinopathy study based on the fundus examination and fluorescein angiography by the same examiner (J.K.C.). CSME was evaluated by slit lamp biomicroscopy using a noncontact lens, as defined by the early treatment diabetic retinopathy study and the extent were assessed with fluorescein angiography and optical coherence tomography (OCT; Zeiss-Humphrey, Dubin, CA). This study included patients with PDR who required PRP and excluded patients with previous intraocular operations such as laser photocoagulation or IVTA, glaucoma, and cataract causing decrease in visual acuity. In addition, to eliminate the effects of systemic conditions, patients who had systolic and diastolic blood pressures of higher than 130 and 80 mmHg respectively, regardless of medication at each visit, and those who were diagnosed diabetic nephropathy by internal medicine specialist were excluded from this study. Glycated hemoglobin levels just before and 3 months after treatment were measured and any patients with levels exceeding 8.0% were excluded from the study. All medical procedures followed the tenets of the Declaration of Helsinki with the approval of our Institutional Review Broad. All patients who gave written consent to this study after complete description of the procedure were randomly allotted. Patients in combined treatment group underwent IVTA with PRP, and patients in laser treatment group underwent focal or grid laser therapy with PRP. At 2 weeks, 1, 2, and 3 months postoperatively, the absence or presence of complications was confirmed by anterior segment examination, fundoscopy, and tonometry, and the outcomes were measured with BCVA, fluorescein angiography and foveal thickness by OCT.
TA (Tamceton; 40 mg/1 ml, Hanall Pharmaceutical, Seoul, Korea) was injected into the vitreous body at the operating theater at the day of surgery after PRP was performed at the lower half of the retina. Since several reports have shown that the vehicle of TA may have toxicity to the intraocular tissue and not a few studies have suggested that TA should be injected after discarding its vehicle,12,13 we prepared TA in the following manner. A vial of TA was set aside until the TA was submerged to the bottom, and the solvent was discarded. Then, the TA was mixed with balanced salt solution (BSS), to make the final volume to 1 ml. From the solution, 0.1 ml was placed in a 1 ml tuberculin syringe and the syringe was placed upside down for 10 minutes to obtain the secondary precipitate. After sterilization of peripalpebral region and conjunctiva with 10% and 5% povidone-iodine solution, proparacaine was instilled into the conjunctiva for topical anesthesia. To minimize the volume effect only the sedimented TA (4 mg) with minimal BSS was injected into the vitreous body until TA crystals disappeared completely at the needle hub (about 0.03~0.05 ml). It was injected 3.0 to 3.5 mm posteriorly from the corneal limbus at five o'clock using a 30 gauge needle.
The injection site was compressed using a cotton pledget for 20 seconds without anterior chamber paracentesis. It was confirmed that there were no complications such as retinal tear and vitreous hemorrhage by fundoscopy using an indirect ophthalmoscope after IVTA. Laser photocoagulation was repeated at the upper half of the retina 2 weeks later.
After retinal photocoagulation was performed at the primary site in combination with focal or grid laser therapy, PRP was conducted three times at 1 week intervals. Two patients underwent grid laser photocoagulation according to the ETDRS guide-lines. Photocoagulation in spots of 100 to 200 µm diameter in the macular area, at a distance of one to two spots from one to another, in concentric lines, with an exposure time of 0.2~0.5 seconds, was performed, with sparing of the central area. Eight patients with microaneurysms ranging from 500 to 3000 µm from the center of the macula were treated with focal laser photocoagulation. The spot size, power, and time setting were 100 µm, 150 mW, and 0.1 seconds. Panretinal scatter photocoagulation in both groups were performed with the size of the spots on the retina was 500 µm, and the duration of the application was 0.15 to 0.2 seconds. A krypton red laser was used, resulting in gray retina. Each spot was produced by a 120 to 200 mW exposure. The total number of burns after complete sessions was 1574.3±41.7 in combined treatment group and 1595.7±56.2 in laser treatment group (p=0.436). Topical anesthesia was used in all cases, and all patients were treated by the same ophthalmologist (K.S.C).
Foveal thickness was taken to calculate the averaged thickness in the central ring, 1000 µm in diameter, by means of the Fast Macular Thickness scan of OCT. BCVA was measured on a Snellen chart after refraction and correction. Analyses of BCVA were performed by converting Snellen visual acuity measurement to log minimum angle of resolution (logMAR) equivalents. Intraocular pressure was measured with a Goldman applanation tonometer.
The Mann-Whitney test and the Wilcoxon signed rank test were applied to compare the differences between the two groups and in the group respectively. The Kruskal Wallis test was used for comparison of three or more parameters. P<0.05 was considered significant, and SPSS for Windows (SPSS Inc., Chicago, IL) was used for statistical analysis.
Ten patients (10 eyes) treated with combination treatment and 10 patients (10 eyes) treated with laser photocoagulation were enrolled for main outcome measurement. The baseline characteristics of the two groups are presented in Table 1.
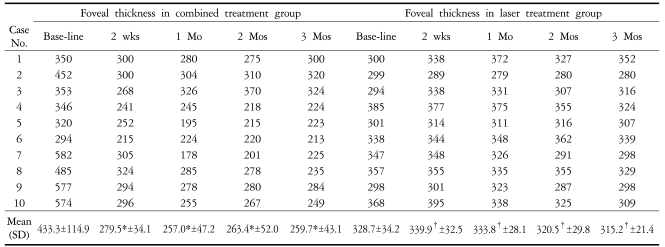
In the group with a combination treatment of IVTA and PRP, postoperative thickness of the fovea decreased significantly to 279.5±34.1 (P=0.005), 257.0±47.2 (P=0.005), 263.4±52.0 (P=0.007), and 259.7±43.1 µm (P=0.005) at 2 weeks, 1, 2, and 3 months, respectively, as compared with preoperative thickness of the fovea (Table 2, Fig. 1). However, there was no significant difference in thickness of the fovea between the experimental time points (P>0.750). In the group with focal or grid laser photocoagulation and PRP, postoperative thickness of the fovea increased to 339.9±32.5 and 333.8±28.1 µm at 2 weeks and 1 month, respectively, as compared with baseline thickness of the fovea without significant difference (P=0.139, and 0.798, respectively), and the macular edema began to decrease 2 months after treatment without significant difference (Table 2, Fig. 2). In the group with a combination treatment, BCVA did not change significantly at 2 weeks after surgery. However, it improved significantly at 1 month (P=0.042) and visual improvement was continued until 3 months (Fig. 1). In the group with laser photocoagulation, visual acuity decreased to 0.50±0.13 (P=0.046), 0.47±0.11 (P=0.792), and 0.47±0.08 (P=0.792) at 2 weeks, 1, and 2 months after treatment, respectively. A transient visual disturbance occurred at 2 weeks then returned to initial visual acuity at 3 months after treatment (Fig. 2). The changes in intraocular pressure in the group with a combination treatment were 16.1±4.2, 17.3±3.8, 16.9±5.7, and 16.6±5.4 mmHg at 2 weeks, 1, 2, and 3 months after surgery, and the intraocular pressure did not exceed 21 mmHg. Complications such as endophthalmitis, retinal detachment, or vitreous hemorrhage did not occur in all patients.
In fluorescein angiography, the group with a combination treatment showed a decrease in the late-onset fluorescein leak in the macula and perivascular area (Fig. 3), whereas the group with laser photocoagulation exhibited only a slight decrease in the leak or an increase (Fig. 4).
In the group with a combination treatment, laser photocoagulation at the upper half of the vitreous was not disturbed by triamcimolone particles which were injected 2 weeks before laser photocoagulation since the particles were localized at the lower half of the retina.
In diabetic retinopathy, diabetic macular edema occurs due to leak and accumulation of body fluid or plasma in the outer plexiform layer or inner nuclear layer which were induced by destruction of the inner blood retinal barrier due to microaneurysm or capillaries damaged by hyper-permeability. This is one of the leading causes of decreased visual acuity in diabetic retinopathy along with vitreous hemorrhage. It is reported that macular edema occurs in 10% of patients with diabetic retinopathy, and the incidence becomes higher with increasing duration and severity of the disease.1-4,14
It has been well known that PRP is effective in the prevention of visual loss and neovascularization.3,4,15,16 However, it is reported that PRP may cause visual loss, macular edema, and aggravation of preexisting macular edema, and the visual loss due to PRP-induced macular edema occurs in 25% to 43% of the patients.6,7,16-19 These problems frequently make ophthalmologists hesitate to perform PRP in patients with both severe diabetic retinopathy and CSME, although the patients have disturbance of everyday life due to visual loss especially in patients with preexisting visual loss.
Gardner et al20 reported that postoperative aggravation of macular edema can be diminished by performing PRP in multiple stages. Aiello et al21 advocated that it is advisable to perform PRP after macular edema is treated by focal or grid laser photocoagulation. However, the therapeutic effect of PRP is limited, and it is not recommended to delay PRP in high-risk PDR.21,22 According to the results of Shimura et al8, when PRP was performed four times at intervals of 1 to 2 weeks on patients with nonproliferative diabetic retinopathy or non-high-risk PDR, transient macular edema was developed in both the group treated at 1 week intervals (1 week group) and the group treated at 2 week intervals (2 week group), indicating that macular edema was more severe in 1 week group than in 2 week group and the maintenance rate of visual acuity at the first visit was lower in 1 week group (89%) than in 2 week group (92%). However, since laser photocoagulation at 2 week intervals requires a total of 8 weeks and the outcomes after PRP are not evaluated yet in PDR patients with macular edema, it is thought to be not an advisable treatment modality.
In the present study, macular edema began to decrease from 2 weeks and was maintained until 3 months, and visual acuity began to improve without transient decrease in visual acuity from 1 month after surgery and was maintained until 3 months in the group with a combination treatment.
In addition, there was no patient whose thickness of the fovea was aggravated again to the preoperative status at each experimental time point (2 weeks, 1, 2, and 3 months). The results from thickness of the macula before and after PRP were presented in patients with milder diabetic retinopathy (nonproliferative diabetic retinopathy or non-high-risk PDR without CSME) as compared to this study by Shimura et al8, who reported that in 1 week group, thickness of the fovea increased 1.42 (±0.05) times more at 4 weeks after PRP than initial foveal thickness, and in 2 week group, it increased 1.16 (±0.03) times more at 7 weeks after PRP than initial foveal thickness. In this study, the results from the group with laser photocoagulation revealed that thickness of the fovea increased 1.034 times and 1.015 times more at 2 weeks and 1 month after treatment, respectively, and 7 of 10 eyes had aggravated macular edema at 2 weeks, six eyes at 1 month, and five eyes at 2 or 3 months after treatment. From these results, it is suggested that a combination of IVTA and PRP is effective in the treatment of diabetic macular edema and prevention of its aggravation after laser photocoagulation. A more important finding in this study was that there was no patient with aggravation of visual acuity after a combination treatment during the follow-up period. Shimura et al9 reported that patients with normal visual acuity of 20/20 or more exhibited transient decrease in visual acuity in 4.7% of patients and persistent decrease in visual acuity of 2 lines or more in 10.9% of patients during the follow-up period of 6 months after PRP. They also reported that occurrence rate of macular edema with subsequent decrease in visual acuity was significantly higher in eyes with a greater parafoveal thickness (> 300 µm) before PRP. Although all patients with a combination treatment except one (case 6) had greater (> 300 µm) parafoveal thickness (401.7±41.5 µm) in the present study, there was no case of aggravation of macular edema or deterioration of visual acuity due to the treatment.
Complications of IVTA include cataract, elevated intraocular pressure, choroidal hemorrhage, retinal detachment, and endophthalmitis.7,23-25 They did not occurred in the group with combination treatment during the follow-up period of 3 months.
After a combination treatment, late fluorescein angiographs exhibited decreased leak in the macular region, which was consistent with the result from OCT, and generalized degeneration of the newly formed vessels. On the grounds that TA is effective in suppressing neovascularization in an experimental setting and reducing synthesis of new vessels in the iris and choroid in a clinical setting, it is inferred that the inhibitory effect of both PRP and TA influence the degeneration of the newly formed blood vessels in PDR.26-28 Although the exact mechanism of aggravation of macular edema after PRP has not been elucidated yet, destruction of blood retinal barrier by laser therapy, which is one of the various retinal diseases causing macular edema and some cytokines, such as interleukin-6 and interleukin-8 related to tissue inflammation appears to contribute to its mechanism.8,9 TA inhibits blood retinal barrier damage induced by cytokines or endothelial growth factor and is effective in the treatment of macular edema, which will explain our finding that occurrence of macular edema can be inhibited by stabilization of damaged blood retinal barrier.29,30
In conclusion, the results of this study suggest that a combination of PRP and IVTA may improve visual acuity through inhibition of macular edema induced by laser photocoagulation and have an inhibitory effect on degeneration of the newly formed blood vessels. TA seems to be synergistic with PRP. In combination with PRP, IVTA might be more effective than laser photocoagulation in reducing diabetic macular edema and preventing aggravation of macular edema and transient visual disturbance in patients requiring immediate PRP. Although additional studies on prognosis and treatment outcomes are needed due to a small sample size and a short duration of follow-up in this study, the advantage of a combination treatment should not be ignored. A combination of PRP and IVTA would be expected to be used as a new treatment modality in patients with macular edema secondary to PDR requiring PRP.
References
1. Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003; 136:122–135. PMID: 12834680.

2. Akduman L, Olk RJ. Laser photocoagulation of diabetic macular edema. Ophthalmic Surg Lasers. 1997; 28:387–408. PMID: 9150523.

3. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103:1796–1806. PMID: 2866759.
4. Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 2. Ophthalmology. 1987; 94:761–774. PMID: 3658348.
5. Ferris FL, Podgor MJ, Davis MD. Macular edema in diabetic retinopathy study patients Diabetic Retinopathy Study Group report number 12. Ophthalmology. 1987; 94:754–760. PMID: 3658347.
6. Kleiner RC, Elman MJ, Murphy RP, Ferris FL 3rd. Transient severe visual loss after panretinal photocoagulation. Am J Ophthalmol. 1988; 106:298–306. PMID: 3421291.
7. Meyers SM. Macular edema after scatter laser photocoagulation for proliferative diabetic retinopathy. Am J Ophthalmol. 1980; 90:210–216. PMID: 6158867.

8. Shimura M, Yasuda K, Nakazaya T, et al. Quantifying alterations of macular thickness before and after pan-retinal photocoagulation in patients with severe diabetic retinopathy and good vision. Ophthalmology. 2003; 110:2386–2394. PMID: 14644723.

9. Shimura M, Yasuda K, Nakazawa T, Tamai M. Visual Dysfunction After Panretinal Photocoagulation in Patients With Severe Diabetic Retinopathy and Good Vision. Am J Ophthalmol. 2005; 140:8–15. PMID: 15939392.

10. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003; 121:57–61. PMID: 12523885.

11. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Am J Ophthalmol. 2002; 109:920–927.

12. Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol. 2000; 84:1064–1067. PMID: 10966969.

13. Sakamoto T, Miyazaki M, Hisatomi T, et al. Triamcinolone assisted pars plana vitrectomy improves the surgical procedures and decreases the postoperative blood-ocular barrier breakdown. Graefes Arch Clin Exp Ophthalmol. 2002; 240:423–429. PMID: 12107507.
14. Bresnick GH. Diabetic macular edema: a review. Ophthalmology. 1986; 93:989–997. PMID: 3531959.
15. Diabetic Retinopathy Study Research Group. Diabetic Retinopathy Study. Report 6. Design, methods and baseline results. Invest Ophthalmol Vis Sci. 1981; 21:149–209.
16. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001; 44:156–163. PMID: 11270671.

17. McDonald HR, Schatz H. Macular edema following panretinal photocoagulation. Retina. 1985; 5:5–10. PMID: 4001591.

18. McDonald HR, Schatz H. Visual loss following panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1985; 92:388–393. PMID: 4039432.

19. Higgins KE, Meyers SM, Jaffe MJ, et al. Temporary loss of foveal contrast sensitivity associated with panretinal photocoagulation. Arch Ophthalmol. 1998; 104:997–1003. PMID: 3729795.

20. Gardner TW, Eller AW, Friberg TR. Reduction of severe macular edema in eyes with poor vision after panretinal photocoagulation for proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1991; 229:323–328. PMID: 1916318.

21. Aiello LM. Albert DM, Jakobiec FA, editors. Diagnosis, management and treatment of nonproliferative diabetic retinopathy and macular edema. Principles and Practice of Ophthalmology. 2000. v. 3:2nd ed. Philadelphia: WB Saunders;chap. 138.
22. Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991; 98:766–786. PMID: 2062512.
23. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003; 87:24–27. PMID: 12488256.

24. Jonas JB, Kreissig I, Degenring R. Secondary chronic open angle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003; 121:729–730. PMID: 12742856.
25. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–796. PMID: 14597028.

26. Antoszyk AN, Gottlieb JL, Machemer R, Hatchel DL. The effects of intravitreal triamcinolone acetonide on experimental pre-retinal neovascularization. Graefe Arch Clin Exp Ophthalmol. 1993; 231:34–40.

27. Gillies MC, Simpson JM, Luo W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results. Arch Ophthalmol. 2003; 121:667–673. PMID: 12742844.
28. Jonas JB, Hayler JK, Sofker A, Panda-Jonas S. Regression of neovascular iris vessel by intravitreal injection of crystalline cortisol. J Glaucoma. 2001; 10:284–287. PMID: 11558812.
29. Wilson CA, Berkowitz BA, Sato Y, et al. Treatment with intravitreal steroid reduces blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992; 110:1155–1159. PMID: 1497531.

30. Zacks DN, Johnson MW. Combined intravitreal injection of triamcinolone acetonide and panretinal photocoagulation for concomitant diabetic macular edema and proliferative diabetic retinopathy. Retina. 2005; 25:135–140. PMID: 15689801.

Fig. 1
Evaluation of mean foveal thickness and best corrected visual acuity after combined intravitreal injection of triamcinolone acetonid and panretinal photocoagulation. ⋆: statistically significant difference between initial foveal thickness and each value, *: statistically significant difference between best corrected visual acuity before and after combination treatment.
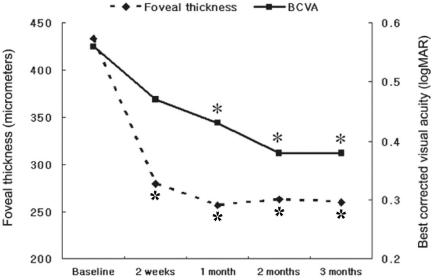
Fig. 2
Changes of foveal thickness and best corrected visual acuity in patients treated with focal or grid laser photocoagulation and panretinal photocoagulation. *: statistically significant difference between best corrected visual acuity before and after laser treatment. The values of foveal thicknessare not significantly different from baseline.
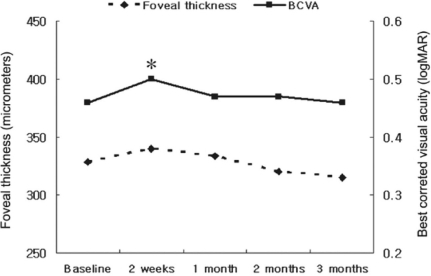
Fig. 3
Fundus photographs (top), fluorescein angiograms (middle), and optical coherence tomography (bottom) of case 7 in combination treatment group. (A) Findings of initial examination. (B) One month after combined intravitreal injection of triamcinolone acetonide and panretinal photocoagulation. Late-phase fluorescein angiogram showing the marked decreased amount of leaking on macula and perivascular area. Note the decreased foveal thickness as seen on the optical coherence tomography.
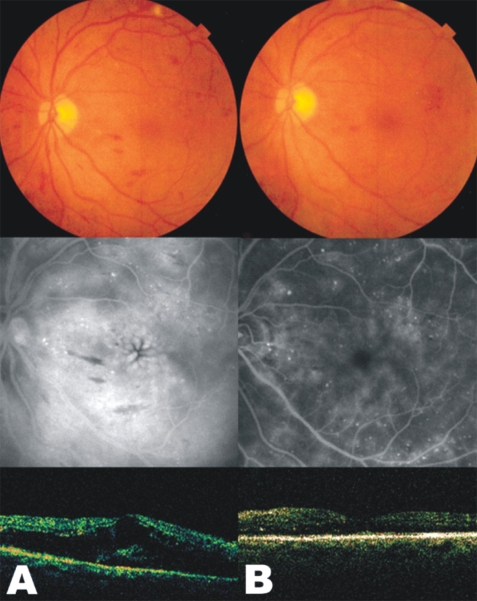
Fig. 4
Fundus photographs (top), fluorescein angiograms (middle), and optical coherence tomography (bottom) of case 1 in laser treatment group. (A) Findings of initial examination. (B) One month after grid laser photocoagulation and panretinal photocoagulation. Late-phase fluorescein angiogram showing increased amount of fluid collection on macula. Note the increased foveal thickness as seen on the optical coherence tomography in spite of laser treatment.
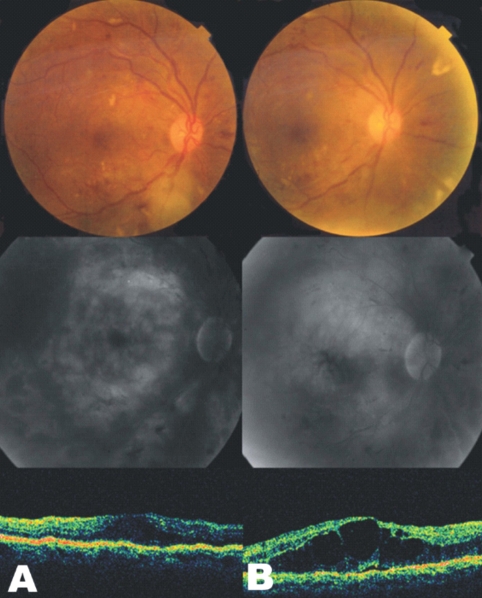
Table 1
Baseline characteristics of two groups enrolled in the present study
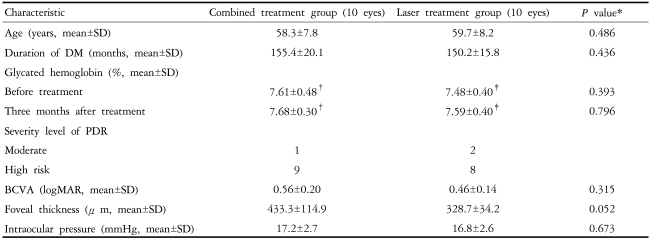
SD: standard deviation, DM: diabetes mellitus, PDR: proliferative diabetic retinopathy, BCVA: best corrected visual acuity, logMAR: log of minimum angle of resolution, *: statistical significance was tested by Mann-Whitney Test, †: p=0.975 by Wilcoxon Singed Ranks Test, ‡: p=0.341 by Wilcoxon Singed Ranks Test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download