Abstract
Purpose
We evaluated the clinical manifestations of varicella-zoster virus (VZV)-induced endotheliitis and treatment outcomes.
Methods
We retrospectively reviewed the medical records of patients exhibiting clinical manifestations of endotheliitis diagnosed as VZV endotheliitis via polymerase chain reaction (PCR) of anterior chamber puncture fluid from January 2013 to December 2018. Their clinical characteristics, treatments, and outcomes were analyzed.
Results
Seven eyes of seven patients were diagnosed as VZV-affected via PCR of the aqueous humor. Mean patient age was 70.4 ± 10.4 years and the average follow-up time 24.7 ± 3.8 months. All eyes exhibited mild anterior chamber inflammation (trace to 1+). Four eyes were disciform in shape and three exhibited diffuse endotheliitis. Six patients evidenced intraocular pressures >21 mmHg. All patients were treated with oral antiviral agents; they were cured and no recurrence was noted. The mean best-corrected visual acuity (logMAR) increased significantly from 0.73 ± 0.19 to 0.09 ± 0.07 and the mean ocular pressure decreased significantly from 26.1 ± 7.3 to 13.2 ± 2.1 mmHg.
Conclusions
VZV endotheliitis may present as mild inflammation of the anterior chamber with a disciform eye or diffuse corneal edema. Diagnosis is aided by VZV-specific PCR of anterior chamber fluid; oral antiviral agents are useful. Be diagnosed with PCR of anterior chamber, and be treated with oral antiviral agents.
Figures and Tables
Figure 1
Clinical photographs of patients 5. Slit lamp photograph showed corneal edema and multiple keratic precipitates at initial visit (A). Slit lamp photograph showed decreased corneal edema and keratic precipitates after the antiviral treatment (B). Corneal central thickness decreased from 632 µm to 558 µm after the treatment (C, D). Decreased number, polymegathism, and hypo-reflectivity of corneal endothelial cells were observed in specular microscopy (E). Multiplex polymerase chain reaction showed positive with VZV in aqueous humor (F). CD = cell density; CMV = cytomegalovirus; HHV6 = human herpes virus 6; EBV = Epstein-Barr virus; VZV = Varicella-zoster virus; HSV1, 2 = herpetic simplex virus 1, 2; IC = internal control; PC = positive control; NC = negative control.
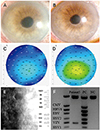
Figure 2
Initial slit lamp photographs of patients 1 to 4. Slit lamp photograph showed diffuse corneal edema and keratic precipitates in the eye of patient 1 with paralytic mydriasis (A, B). Disciform corneal edema and mutton-fat keratic precipitates were observed in the eye of patient 2 with paralytic mydriasis (C, D). Iris atrophy and keratic precipitates were observed in the eye of patient 3 (E, F). Disciform corneal edema and pseudodendritic lesion, keratic precipitates were observed in the eye of patient 4 (G, H).
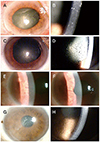
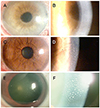
Figure 3
Initial slit lamp photographs of patients 5 to 7. Slit lamp photograph showed diffuse corneal edema and mutton-fat keratic precipitates in the eye of patient 5 (A, B). Corneal edema and keratic precipitates were observed in the eye of patient 6 (C, D). Disciform corneal edema and mutton-fat keratic precipitates were observed in the eye of patient 7 (E, F).
References
1. Suzuki T, Ohashi Y. Corneal endotheliitis. Semin Ophthalmol. 2008; 23:235–240.
2. Koizumi N, Yamasaki K, Kawasaki S, et al. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am J Ophthalmol. 2006; 141:564–565.
3. Koizumi N, Inatomi T, Suzuki T, et al. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan corneal endotheliitis study. Br J Ophthalmol. 2015; 99:54–58.
4. Khodabande A. Varicella endotheliitis: a case report. Eur J Ophthalmol. 2009; 19:1076–1078.
5. de Freitas D, Sato EH, Kelly LD, Pavan-Langston D. Delayed onset of varicella keratitis. Cornea. 1992; 11:471–474.
6. Sutcliffe E, Baum J. Acute idiopathic corneal endotheliitis. Trans Am Ophthalmol Soc. 1983; 81:86–96.
7. Peng RM, Guo YX, Xiao GG, et al. Clinical manifestations and characteristics of in vivo confocal microscopy in varicella zoster virus-related corneal endotheliitis. Ocul immunol inflamm. 2019; 27:1270–1279.
8. Kim YJ, Yoo WS, Han YS, et al. Clinical manifestations and outcomes of treatment in cytomegalovirus endotheliitis. J Korean Ophthalmol Soc. 2016; 57:863–875.
9. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008; 115(2 Suppl):S3–S12.
10. Bandeira F, Roizenblatt M, Levi GC, et al. Herpes zoster ophthalmicus and varicella zoster virus vasculopathy. Arq Bras Oftalmol. 2016; 79:126–129.
11. Mun CY, Jung MS. Clinical features and risk factors of herpes zoster ophthalmicus. J Korean Ophthalmol Soc. 2017; 58:1317–1324.
12. Takase H, Kubono R, Terada Y, et al. Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol. 2014; 58:473–482.
13. Sakai JI, Usui Y, Suzuki J, et al. Clinical features of anterior uveitis caused by three different herpes viruses. Int Ophthalmol. 2019; 05. 27. DOI: 10.1007/s10792-019-01125-5. [Epub ahead of print].
14. Tugal-Tutkun I, Cimino L, Akova YA. Review for disease of the year: varicella zoster virus-induced anterior uveitis. Ocul Immunol inflamm. 2018; 26:171–177.
15. Huff JC, Bean B, Balfour HH Jr, et al. Therapy of herpes zoster with oral acyclovir. Am J Med. 1988; 85:84–89.
16. McKendrick MW, McCil JI, White JE, Wood MJ. Oral acyclovir in acute herpes zoster. Br Med J (Clin Res Ed). 1986; 293:1529–1532.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download