Abstract
Purpose
To evaluate the effectiveness of topical steroid instillation and associated factors in patients with recently developed nasolacrimal drainage stenosis.
Methods
The medical records of 108 eyes of 56 patients who received topical steroid for nasolacrimal drainage stenosis between January 2010 and June 2013 and who developed epiphora of a three-month duration were retrospectively reviewed. Evaluations were performed at 1, 3, and 6 months after instillation of topical steroid. Subjective symptoms, tear meniscus height, and fluorescein dye disappearance test results were noted at every follow-up visit; nasolacrimal irrigation and canalicular probing were also performed as needed.
Results
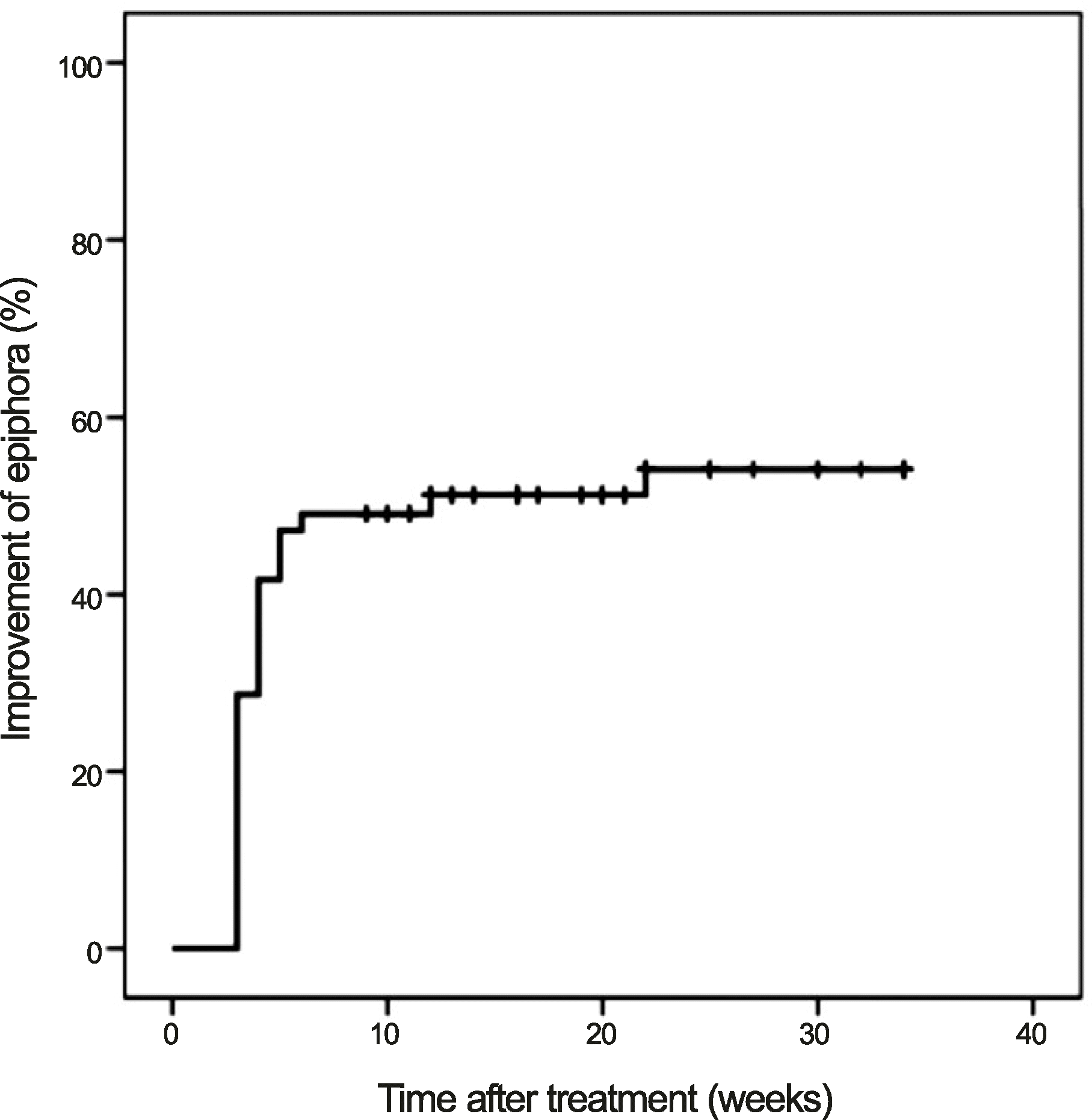
The cumulative improvements were 42.0% at 1 month and 51.0% at 3 months after treatment, and the mean period of symptomatic improvement was 3.8 ± 1.9 weeks (range, 3-12 weeks). At the final follow-up visit, 56 (51.9%) of 108 eyes showed improved epiphora. Final improvement was observed in 63% (12 of 19 eyes) of idiopathic nasolacrimal drainage stenosis patients, in 100% (10 of 10 eyes) of docetaxel-treated patients, and in 43% (34 of 79 eyes) of S-1-treated patients.
References
1. Sibley D, Norris JH, Malhotra R. Management and outcomes of patients with epiphora referred to a specialist ophthalmic plastic unit. Clin Experiment Ophthalmol. 2013; 41:231–8.

2. Tannenbaum M, McCord CD. The lacrimal drainage system. Tasman W, Jaeger EA, editors. Duane's Clinical Ophthalmology. Philadelphia: JB Lippincott Co.;1991. 4:chap. 13. p. 1–33.
3. Linberg JV, McCormick SA. Primary acquired nasolacrimal duct obstruction. A clinicopathologic report and biopsy technique. Ophthalmology. 1986; 93:1055–63.
4. Lee-Wing MW, Ashenhurst ME. Clinicopathologic analysis of 166 patients with primary acquired nasolacrimal duct obstruction. Ophthalmology. 2001; 108:2038–40.

5. Mandeville JT, Woog JJ. Obstruction of the lacrimal drainage system. Curr Opin Ophthalmol. 2002; 13:303–9.

6. Dayal Y. CORTICOSTEROIDS AND FIBROLYSIN IN THE PREVENTION OF LACRIMAL DUCT OBSTRUCTION. Br J Ophthalmol. 1962; 46:27–30.

7. Esmaeli B, Hidaji L, Adinin RB, et al. Blockage of the lacrimal drainage apparatus as a side effect of docetaxel therapy. Cancer. 2003; 98:504–7.

8. Hirschbein MJ, Stasior GO. Lacrimal system. Chen WP, editor. Oculoplastic surgery: The Essentials. 1st ed.New York: Thieme Medical Publishers, Inc;2001. p. 263–88.
9. Vick VL, Holds JB, Hartstein ME, Massry GG. Tarsal strip procedure for the correction of tearing. Ophthal Plast Reconstr Surg. 2004; 20:37–9.

10. Woog JJ. The incidence of symptomatic acquired lacrimal outflow obstruction among residents of Olmsted County, Minnesota, 1976-2000 (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007; 105:649–66.
11. Ohtomo K, Ueta T, Toyama T, Nagahara M. Predisposing factors for primary acquired nasolacrimal duct obstruction. Graefes Arch Clin Exp Ophthalmol. 2013; 251:1835–9.

12. Esmaeli B, Valero V, Ahmadi MA, Booser D. Canalicular stenosis secondary to docetaxel (taxotere): a newly recognized side effect. Ophthalmology. 2001; 108:994–5.
13. Esmaeli B, Ahmadi MA, Rivera E, et al. Docetaxel secretion in tears: association with lacrimal drainage obstruction. Arch Ophthalmol. 2002; 120:1180–2.
14. Esmaeli B, Hortobagyi GN, Esteva FJ, et al. Canalicular stenosis secondary to weekly versus every-3-weeks docetaxel in patients with metastatic breast cancer. Ophthalmology. 2002; 109:1188–91.

15. Esmaeli B, Amin S, Valero V, et al. Prospective study of incidence and severity of epiphora and canalicular stenosis in patients with metastatic breast cancer receiving docetaxel. J Clin Oncol. 2006; 24:3619–22.

16. Kintzel PE, Michaud LB, Lange MK. Docetaxel-associated epiphora. Pharmacotherapy. 2006; 26:853–67.

17. Leyssens B, Wildiers H, Lobelle JP, et al. A double-blind randomized phase II study on the efficacy of topical eye treatment in the prevention of docetaxel-induced dacryostenosis. Ann Oncol. 2010; 21:419–23.

18. Prasad S, Kamath GG, Phillips RP. Lacrimal canalicular stenosis associated with systemic 5-fluorouacil therapy. Acta Ophthalmol Scand. 2000; 78:110–3.
19. Eiseman AS, Flanagan JC, Brooks AB, et al. Ocular surface, ocular adnexal, and lacrimal complications associated with the use of systemic 5-fluorouracil. Ophthal Plast Reconstr Surg. 2003; 19:216–24.

20. McCartney E, Valluri S, Rushing D, Burgett R. Upper and lower system nasolacrimal duct stenosis secondary to paclitaxel. Ophthal Plast Reconstr Surg. 2007; 23:170–1.

21. Kim N, Park C, Park DJ, et al. Lacrimal drainage obstruction in gastric cancer patients receiving S-1 chemotherapy. Ann Oncol. 2012; 23:2065–71.

22. Lazic R, Lukic M, Boras I, et al. Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina. 2014; 34:719–24.

23. Kuchar A, Lukas J, Steinkogler FJ. Bacteriology and antibiotic therapy in congenital nasolacrimal duct obstruction. Acta Ophthalmol Scand. 2000; 78:694–8.

24. Notivol R, Bertin D, Amin D, et al. Comparison of topical to-bramycin-dexamethasone with dexamethasone-neomycin-polymyxin and neomycin-polymyxin-gramicidin for control of inflammation after cataract surgery: results of a multicenter, prospective, three-arm, randomized, double-masked, controlled, parallel-group study. Clin Ther. 2004; 26:1274–85.

25. DeAngelis D, Hurwitz J, Mazzulli T. The role of bacteriologic infection in the etiology of nasolacrimal duct obstruction. Can J Ophthalmol. 2001; 36:134–9.

26. Hudis CA, Seidman AD, Crown JP, et al. Phase II and pharmacologic study of docetaxel as initial chemotherapy for metastatic breast cancer. J Clin Oncol. 1996; 14:58–65.

27. Esmaeli B, Burnstine MA, Ahmadi MA, Prieto VG. Docetaxel-induced histologic changes in the lacrimal sac and the nasal mucosa. Ophthal Plast Reconstr Surg. 2003; 19:305–8.

28. Esmaeli B, Golio D, Lubecki L, Ajani J. Canalicular and nasolacrimal duct blockage: an ocular side effect associated with the antineoplastic drug S-1. Am J Ophthalmol. 2005; 140:325–7.

29. Kitamura H, Miyanaga T, Shin H, et al. [Investigation of epiphora following S-1 therapy]. Gan To Kagaku Ryoho. 2011; 38:259–62.
30. ARMALY MF. STATISTICAL ATTRIBUTES OF THE STEROID HYPERTENSIVE RESPONSE IN THE CLINICALLY NORMAL EYE. I. THE DEMONSTRATION OF THREE LEVELS OF RESPONSE. Invest Ophthalmol. 1965; 4:187–97.
Figure 2.
The cumulative incidence of epiphora improvement after treatment of topical steroid, grouped by the etiology of nasolacrimal drainage stenosis. Differences of cumulative incidence of improvement between subgroups were not significant (p = 0.305, log rank test).
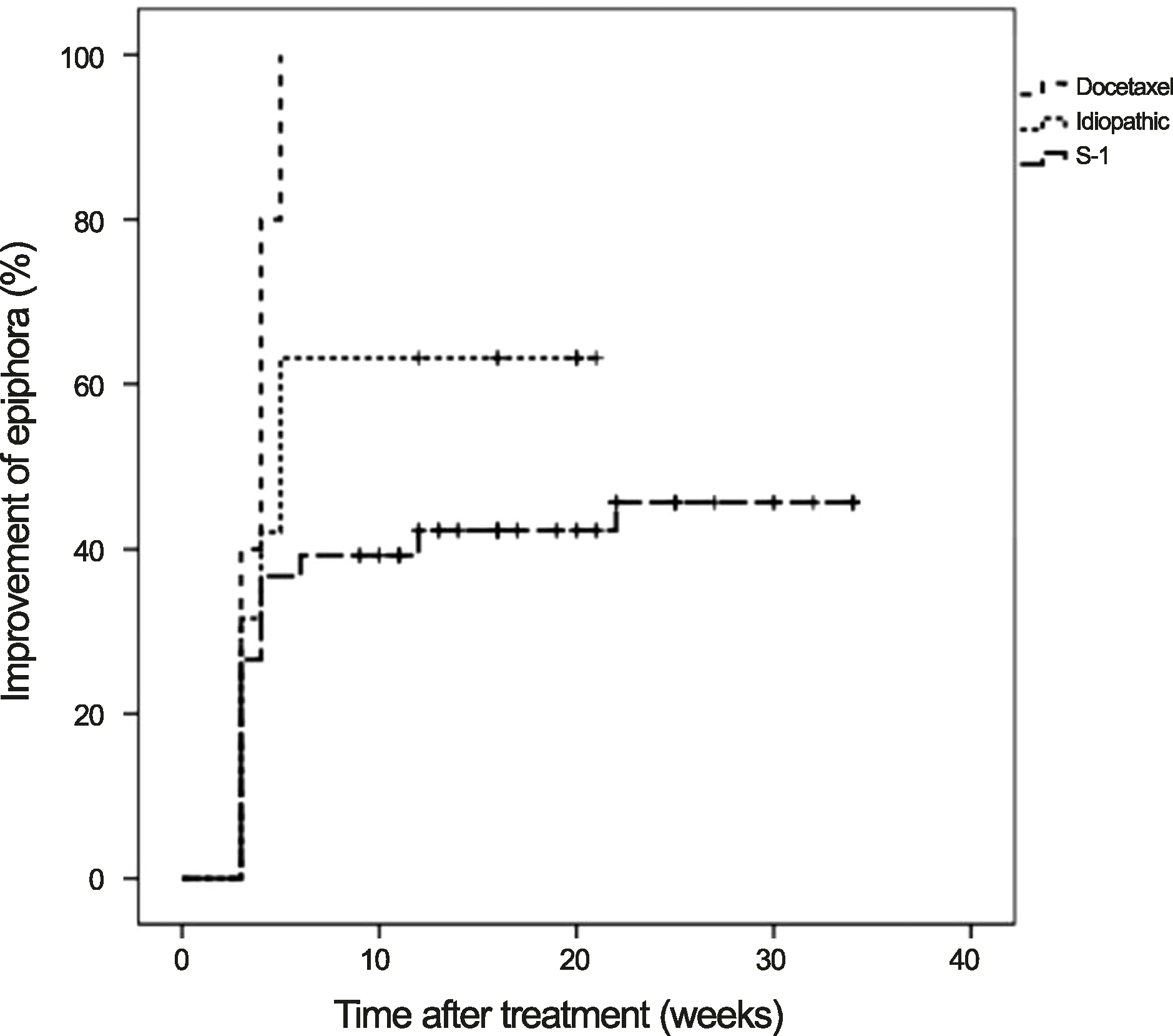
Table 1.
Demographic characteristics of 56 patients (108 eyes) with recently developed nasolacrimal drainage stenosis
Table 2.
Factors affecting improvement of epiphora after instillation of topical steroid in subgroup of idiopathic nasolacrimal drainage stenosis
| Improved | Not improved | p-value* | |
|---|---|---|---|
| (eye, n = 12) | (eye, n = 7) | ||
| Female (eye, %) | 5 (41.7) | 4 (57.1) | 0.650 |
| Dry eye (eye, %) | 4 (33.3) | 4 (57.1) | 0.377 |
| Discharge from the eye (eye, %) | 2 (16.7) | 0 (0.0) | 0.509 |
| Tear meniscus height ≥ moderate degree (eye, %) | 9 (75.0) | 6 (85.7) | 1.000 |
| FDDT ≥ Gr3 (eye, %) | 5 (41.7) | 4 (57.1) | 0.650 |
| Reflux on lacrimal irrigation (eye, %) | 2 (16.7) | 4 (57.1) | 0.129 |
| NLD dilation on DCG (eye, %) | 6 (50.0) | 4 (57.1) | 0.656 |
| Canalicular stenosis (eye, %) | 1 (8.3) | 1 (14.3) | 1.000 |
| Development of epiphora in winter (eye, %) | 5 (41.7) | 3 (42.9) | 1.000 |
Table 3.
Factors affecting improvement of epiphora after instillation of topical steroid in subgroup of S-1 related nasolacrimal drainage stenosis
| Improved | Not improved | p-value* | |
|---|---|---|---|
| (eye, n = 34) | (eye, n = 45) | ||
| Female (eye, %) | 16 (47.1) | 7 (15.6) | 0.003 |
| Dry eye (eye, %) | 14 (41.2) | 12 (26.7) | 0.228 |
| Discharge from the eye (eye, %) | 12 (35.3) | 16 (35.6) | 1.000 |
| Tear meniscus height ≥ moderate degree (eye, %) | 29 (85.3) | 34 (75.6) | 0.399 |
| FDDT ≥ Gr3 (eye, %) | 9 (26.5) | 20 (44.4) | 0.157 |
| Reflux on lacrimal irrigation (eye, %) | 14 (41.2) | 16 (35.6) | 0.133 |
| NLD dilation on DCG (eye, %) | 17 (50.0) | 29 (64.4) | 1.000 |
| Canalicular stenosis (eye, %) | 3 (8.8) | 7 (15.6) | 0.502 |
| Epiphora development within 3 months of chemotherapy (eye, %) | 20 (58.8) | 27 (60.0) | 1.000 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download