Abstract
Purpose
To compare the choroidal thickness in central serous chorioretiopathy (CSC) patients and normal controls using spectral domain optical coherence tomography (SD-OCT).
Methods
The authors compared the choroidal thickness in eyes with CSC, fellow eyes and in normal eyes. In addition, the authors attempted to determine any correlation between choroidal thickness and other factors such as age, height of serous retinal detachment, and spherical equivalent. Choroidal thickness was measured using a perpendicular line from the outer margin of the subfoveal retinal pigment epithelium to the inner surface of the sclera.
Results
Twenty-five eyes of 25 CSC patients, 17 fellow eyes and 29 age-matched normal eyes were examined and categorized as group 1, group 2 and group 3, respectively. Subfoveal choroidal thickness was 370.64 ± 58.06 µm in group 1, 301.85 ± 47.83 µm in group 2, and 261.84 ± 48.22 µm in group 3. The choroidal thickness in group 1 was significantly greater than those in group 2 and group 3, and the choroidal thickness in group 2 was significantly greater than that in group 3 (p = 0.001, p < 0.001, p = 0.004, respectively), where the choroidal thickness showed a negative correlation with age (p = 0.015).
Figures and Tables
Figure 1
Representative images of both eyes in 40-year-old female patient with central serous chorioretinopathy. The choroid is seen in cross-section image acquired by Cirrus HD-OCT high-definition single-line raster scan mode. Perpendicular line was drawn from the outer border of the retinal pigment epithelium to the inner border of the sclera using Cirrus HD-OCT software. Subfoveal choroidal thickness was measured. Choroidal thickness was 379 µm in an eye of central serous chorioretinopathy (A) and 264 µm in an asymptomatic fellow eye (B).
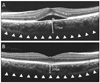
Figure 2
Scatter plot of subfoveal choroidal thickness and age. In eyes with central serous chorioretinopathy (left), the choroidal thickness did not correlate with patient age (Spearman's correlation test, r = 0.276, p = 0.182). In normal eyes (right), however, the choroidal thickness showed negative correlation with age (Spearman's correlation test, r = -0.449, p = 0.015).

Table 2
Comparison of subfoveal choroidal thickness in three groups

Group 1: eyes with central serous chorioretinopathy; Group 2: asymptomatic fellow eyes of central serous chorioretinopathy; Group 3: normal eyes.
*Wilcoxon signed rank test, p-value of group 1 and group 2; †Mann-Whitney U-test, p-value of group 1 and group 3; ‡Mann-Whitney U-test, p-value of group 2 and group 3.
References
1. Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986. 224:321–324.
2. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967. 63:1–139.
3. Tittl M, Polska E, Kircher K, et al. Topical fundus pulsation measurement in patients with active central serous chorioretinopathy. Arch Ophthalmol. 2003. 121:975–978.
4. Tittl M, Maar N, Polska E, et al. Choroidal hemodynamic changes during isometric exercise in patients with inactive central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2005. 46:4717–4721.
5. Iida T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999. 19:508–512.
6. Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996. 121:26–34.
7. Giovannini A, Scassellati-Sforzolini B, D'Altobrando E, et al. Choroidal findings in the course of idiopathic serous pigment epithelium detachment detected by indocyanine green videoangiography. Retina. 1997. 17:286–293.
8. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009. 29:1469–1473.
9. Alam S, Zawadzki RJ, Choi S, et al. Clinical application of rapid serial fourier-domain optical coherence tomography for macular imaging. Ophthalmology. 2006. 113:1425–1431.
10. Ojima Y, Hangai M, Sasahara M, et al. Three-dimensional imaging of the foveal photoreceptor layer in central serous chorioretinopathy using high-speed optical coherence tomography. Ophthalmology. 2007. 114:2197–2207.
11. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008. 146:496–500.
12. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009. 147:811–815.
13. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010. 117:1792–1799.
14. Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010. 150:325–329.
15. Chen TC, Cense B, Miller JW, et al. Histologic correlation of in vivo optical coherence tomography images of the human retina. Am J Ophthalmol. 2006. 141:1165–1168.
16. Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009. 147:801–810.
17. Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009. 147:644–652.
18. Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009. 148:445–450.
19. Spaide RF, Hall L, Haas A, et al. Indocyanine green video-angiography of older patients with central serous chorioretinopathy. Retina. 1996. 16:203–213.
20. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996. 103:2070–2079.
21. Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984. 68:815–820.
22. Lu JG, Friberg TR. Idiopathic central serous retinopathy in China: a report of 600 cases (624 eyes) treated by acupuncture. Ophthalmic Surg. 1987. 18:608–611.
23. Bujarborua D, Chatterjee S, Choudhury A, et al. Fluorescein angiographic features of asymptomatic eyes in central serous chorioretinopathy. Retina. 2005. 25:422–429.
24. Kitzmann AS, Pulido JS, Diehl NN, et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008. 115:169–173.
25. Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992. 81:379–386.
26. Payne GW, Bearden SE. The microcirculation of skeletal muscle in aging. Microcirculation. 2006. 13:275–277.
27. Muller-Delp JM. Aging-induced adaptations of microvascular reactivity. Microcirculation. 2006. 13:301–314.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download