Abstract
Purpose
To evaluate the short-term efficacy of intravitreal ranibizumab injection in eyes with macular edema secondary to central retinal vein occlusion (CRVO).
Methods
The records of 17 patients (17 eyes, 11 ischemic, six ischemic) who received an intravitreal ranibizumab injection for macular edema secondary to CRVO were retrospectively analyzed. The ophthalmic examination included best corrected visual acuity (BCVA) and central macular thickness (CMT) at baseline and follow-up visits.
Results
After intravitreal ranibizumab injection, the mean BCVA improved from log MAR 1.25 ± 0.47 at baseline to log MAR 0.78 ± 0.49 at 4 weeks and to log MAR 0.81 ± 0.52 at 12 weeks (p < 0.05). Additionally, the mean CMT decreased from 679.4 ± 230.5 μ m at baseline to 224.4 ± 129.9 μ m at 4 weeks and to 271.6 ± 174.1 μ m at 12 weeks (p < 0.001). In subgroup analysis, the decreases in CMT at 4 weeks and 12 weeks were similar in ischemic CRVO and non-ischemic CRVO, but no significant changes in visual acuity were found at 12 weeks in the ischemic CRVO group (p = 0.138). Ten eyes (58.8%) did not require reinjections for macular edema for up to 12 weeks.
References
1. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997; 115:486–91.
2. Bashshur ZF, Ma'luf RN, Allam S, et al. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1137–40.
3. Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc. 1981; 79:371–422.
4. Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005; 140:256–61.

5. Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
6. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.

7. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.
8. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab(Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009; 93:452–6.
9. Beutel J, Ziemssen F, Lüke M, et al. Intravitreal bevacizumab treatment of macular edema in central retinal vein occlusion: one-year results. Int Ophthalmol. 2010; 30:15–22.

10. Lazić R, Boras I, Vlasić M, et al. Anti-VEGF in treatment of central retinal vein occlusion. Coll Antropol. 2010; 34:69–72.
11. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

12. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.

13. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–9.

14. Pieramici DJ, Rabena M, Castellarin AA, et al. Ranibizumab for the treatment of macular edema associated with perfused central retinal vein occlusions. Ophthalmology. 2008; 115:e47–54.

15. Spaide RF, Chang LK, Klancnik JM, et al. Prospective study of intravitreal ranibizumab as a treatment for decreased visual acuity secondary to central retinal vein occlusion. Am J Ophthalmol. 2009; 147:298–306.

16. Choi SW, Kim HW, Yun IH. Intravitreal bevacizumab treatment of macular edema in central retinal vein occlusion. J Korean Ophthalmol Soc. 2010; 51:707–15.

17. The Central Vein Occlusion Study Group M report. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology. 1995; 102:1425–33.
18. The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997; 115:486–91.
19. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol. 2001; 411:231–43.

20. Gregori NZ, Rosenfeld PJ, Puliafito CA, et al. One-year safety and efficacy of intravitreal triamcinolone acetonide for the management of macular edema secondary to to central retinal vein occlusion. Retina. 2006; 26:889–95.
21. Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Arch Ophthalmol. 2002; 120:1217–9.

22. Shah NJ, Shah UN. Long-term effect of early intervention with single intravitreal injection of bevacizumab followed by panretinal and macular grid photocoagulation in central retinal vein occlusion (CRVO) with macular edema: A pilot study. Eye. 2011; 25:239–44.

23. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007; 114:2179–82.

24. Rensch F, Jonas JB, Spandau UH. Early intravitreal bevacizumab for non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2009; 87:77–81.

25. Ferrara DC, Koizumi H, Spaide RF. Early bevacizumab treatment of central retinal vein occlusion. Am J Ophthalmol. 2007; 144:864–71.

Figure 1.
Changes of the visual acuity (log MAR) in 11 patients with non-ischemic and 6 patients with ischemic central retinal vein occlusion after intravitreal ranibizumab injection. Mean visual acuity in all patients prior to injection was log MAR 1.25 ± 0.47, Visual acuity increased to log MAR 0.78 ± 0.49, and log MAR 0.81 ± 0.52, 1 and 3 months after ranibizumab injection. CRVO = central retinal vein occlusion.
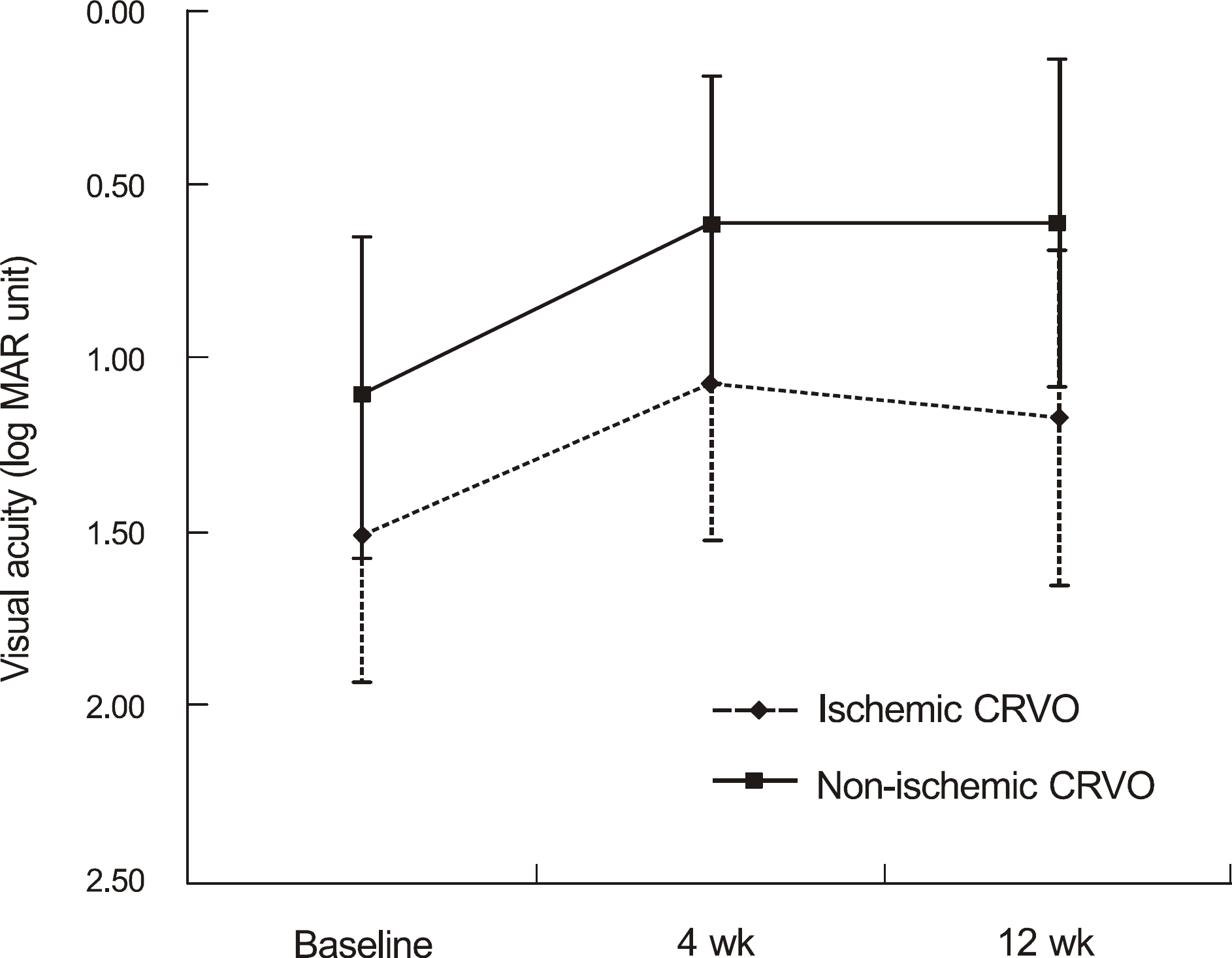
Figure 2.
Changes of the central macular thickness (μ m) measured by optical coherence tomography in 11 patients with non-ischemic and 6 patients with ischemic central retinal vein occlusion after intravitreal ranibizumab injection. Mean central macular thickness in all patients prior to injection was 679.4 ± 230.5 μ m. Central macular thickness decreased to 224.4 ± 129.9 μ m, and 271.6 ± 174.1 μ m, 4 and 12 weeks after ranibizumab injection. CRVO = central retinal vein occlusion.
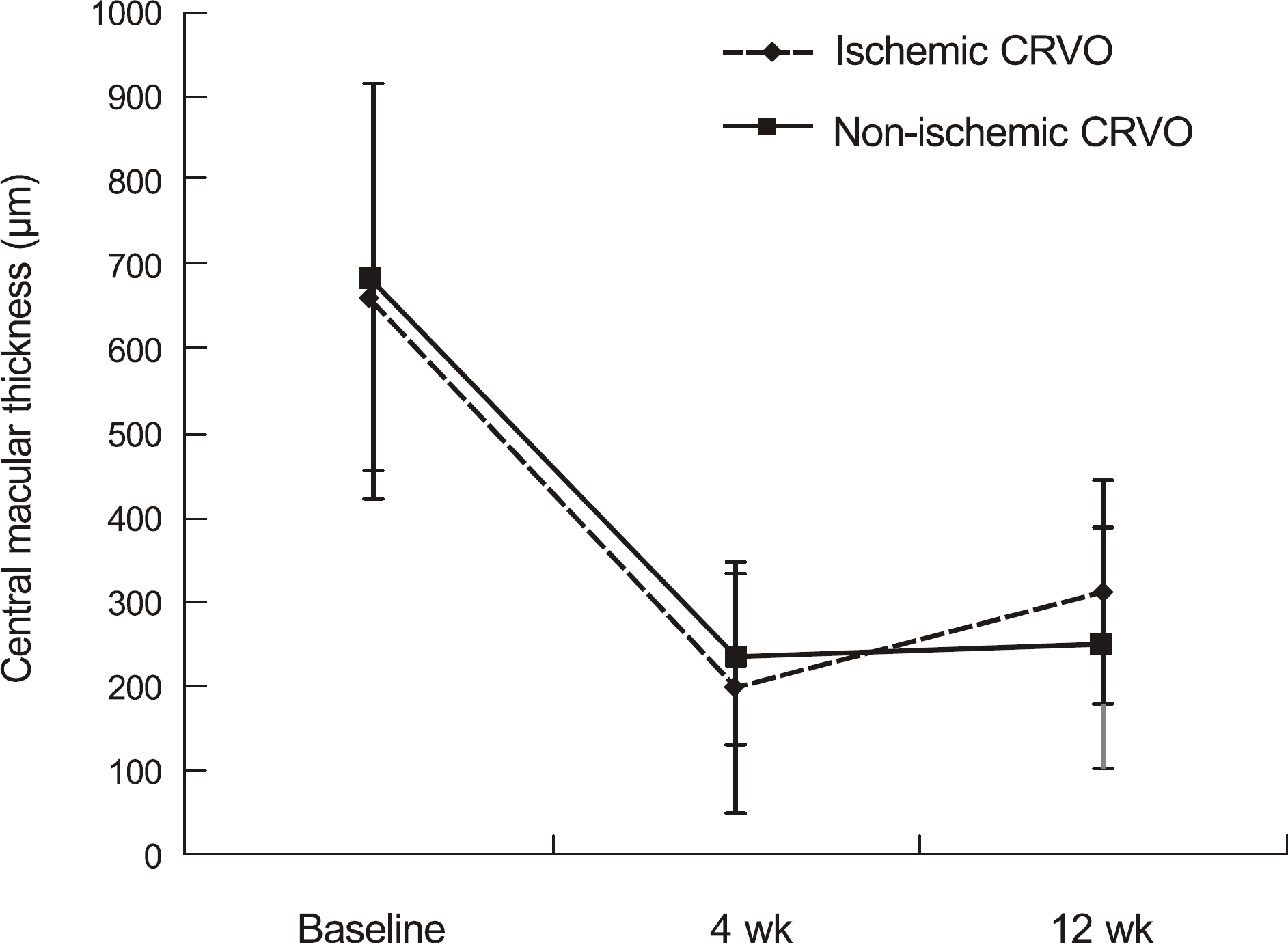
Table 1.
Demographics of patients
| Demographic data | |
|---|---|
| Number of eyes | 17 |
| Sex (M:F) | 11:6 |
| Non-ischemic:ischemic | 11:6 |
| Mean patient age (mean ± SD*, yr) | 60.24 ± 13.22 |
| Baseline BCVA† (mean ± SD, log MAR‡) | 1.25 ± 0.47 |
| Baseline CMT§ (mean ± SD, μ m) | 679.4 ± 230.5 |
Table 2.
Changes in visual acuity after ranibizumab injection (mean ± SD*)
| | | Baseline | 4 weeks | 12 weeks |
|---|---|---|---|---|
| BCVA† (log MAR‡) | Total CRVO§ | 1.25 ± 0.47 | 0.78 ± 0.49 | 0.81 ± 0.52 |
| | | | (p < 0.001) | (p = 0.001) |
| | Non-ischemic CRVO | 1.11 ± 0.46 | 0.62 ± 0.43 | 0.61 ± 0.47 |
| | | | (p = 0.008) | (p = 0.003) |
| | Ischemic CRVO | 1.51 ± 0.42 | 1.07 ± 0.45 | 1.17 ± 0.48 |
| | | | (p = 0.029) | (p = 0.138) |
Table 3.
Changes in central macular thickness after ranibizumab injection (mean ± SD*)
| | | Baseline | 4 weeks | 12 weeks |
|---|---|---|---|---|
| CMT† (μ m) | Total CRVO‡ | 679.4 ± 230.5 | 224.4 ± 129.9 | 271.6 ± 174.1 |
| | | | (p < 0.001) | (p < 0.001) |
| | Non-ischemic CRVO | 685.6 ± 242.5 | 237.2 ± 145.5 | 249.5 ± 132.7 |
| | | | (p = 0.003) | (p = 0.004) |
| | Ischemic CRVO | 668.0 ± 228.4 | 201.0 ± 103.2 | 312.2 ± 142.3 |
| | | | (p = 0.028) | (p = 0.030) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download