Abstract
Purpose
To estimate the annual incidence rate, evaluate any changes, analyze the microbiologic spectrum of infecting organisms, antibiotic susceptibility, and factors associated with visual outcomes of postoperative endophthalmitis following cataract surgery over an 8-year period.
Methods
A retrospective investigation of direction, sex, age, culture results, interval duration, and initial visual acuity of 29 patients with endophthalmitis following cataract surgery was conducted from January 2000 to December 2007. The study was divided into two 4-year periods, with patients categorized into either Group 1 or 2.
Results
The incidence rate was 0.359%, the major infective organism was Staphylococcus epidermidis, and no significant change was observed during the 8-year period. Vancomycin retained efficacy in all cases, but increased resistance occurred with ciprofloxacin during the 8 years. Increased visual acuity after treatment was greater in Group 2 and the interval from onset of ocular symptoms to ophthalmic consultation was shorter in Group 2, although a statistical significance was not demonstrated.
Conclusions
Vancomycin remains effective for patients with endophthalmitis following cataract surgery. Patients who initially had good visual acuity showed greater improvement. However, sex, age, or whether or not the patients had a vitrectomy operation, were not statistically significant factors in the improvement of visual acuity.
References
1. Lalwani GA, Flynn HW Jr, Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996-2005). Ophthalmology. 2008; 115:473–6.

2. Mollan SP, Gao A, Lockwood A, et al. Postcataract endophthalmitis: Incidence and microbial isolates in a United Kingdom region from 1996 through 2004. J Cataract Refract Surg. 2007; 33:265–8.

3. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery. Arch Ophthalmol. 2005; 123:613–20.

4. Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study; a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995; 113:1479–96.
5. Nagaki Y, Hayasaka S, Kadoi C, et al. Bacterial endophthalmitis after small-incision cataract surgery: effect of incision placement and intraocular lens type. J Cataract Refract Surg. 2003; 29:20–6.
6. Cooper BA, Holekamp NM, Bohigian G, Thompson PA. Case-control study of endophthalmitis after cataract surgery comparing scleral tunnel and clear corneal wounds. Am J Ophthalmol. 2003; 136:300–5.

7. Barza M, Pavan PR, Doft BH, et al. Evaluation of microbiological diagnostic techniques in postoperative endophthalmitis in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 1997; 115:1142–50.

8. Sharma S, Jalali S, Adiraju MV, et al. Sensitivity and predictability of vitreous cytology, biopsy, and membrane filter culture in endophthalmitis. Retina. 1996; 16:525–9.

9. Recchia FM, Busbee BG, Pearlman RB. Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch Ophthalmol. 2005; 123:341–6.

10. Schmitz S, Dick HB, Krummenauer F, Pfeiffer N. Endophthalmitis in cataract surgery: Results of a German survey. Ophthalmology. 1999; 106:1869–77.
11. Chang DF, Rosa Braga-Mele, Mamalis N, et al. Prophylaxis of postoperative endophthalmitis after cataract surgery. J Cataract Refract Surg. 2007; 33:1801–5.

12. Starr MB, Lally JM. Antimicrobial prophylaxis for ophthalmic surgery. Surv Ophthalmol. 1995; 39:485–501.
13. Laatikainen L, Tarkkanen A. Early vitrectomy in the treatment of post-operative purulent endophthalmitis. Acta Ophthalmol. 1987; 65:455–60.

14. Chen YJ, Kuo HK, Wu PC, et al. A 10-Year comparison of endogenous endophthalmitis outcomes: An East Asian experience with Klebsiella pneumonia infection. Retina. 2004; 24:383–90.
Figure 1.
Monthly incidence of postoperative endophthalmitis following cataract surgery.* Monthly incidence of treated patients that have postoperative endophthalmitis following cataract surgery at our hospital.
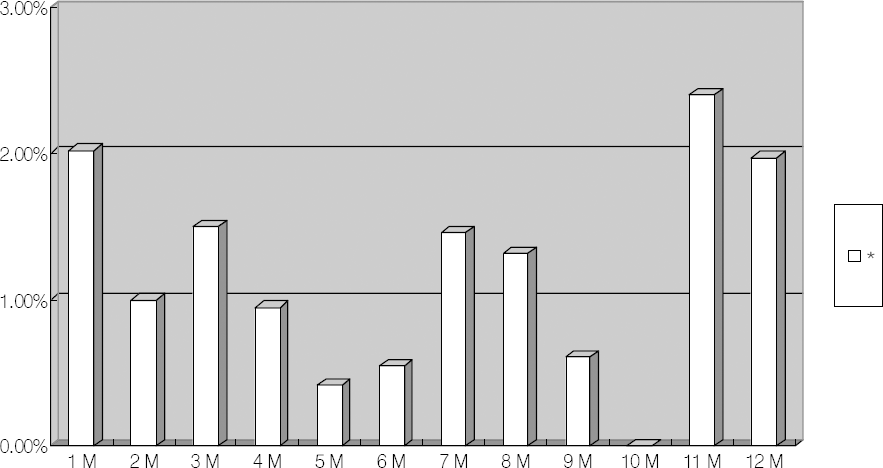
Table 1.
29 Cases of postoperative endophthalmitis following cataract surgery
| Sex/Age | Eye (OD/OS) | Culture | Duration (Days) | Initial VA (Scale)∏ | Final VA (Scale) | Surgery | Year | |
|---|---|---|---|---|---|---|---|---|
| Our Hospital patients | ||||||||
| 1 | F/76 | OS | +* | 3 | 3 | 5 | TPPV§ | 2007.03 |
| 2 | F/71 | OS | + | 2 | 3 | 1 | TPPV | 2007.03 |
| 3 | M/62 | OD | -† | 11 | 5 | 4 | - | 2001.11 |
| 4 | M/70 | OS | - | 5 | 6 | 6 | TPPV | 2003.04 |
| 5 | F/71 | OS | ND‡ | 7 | 3 | 6 | - | 2006.03 |
| 6 | M/65 | OS | ND | 3 | 3 | 5 | - | 2006.01 |
| 7 | F/74 | OS | + | 8 | 4 | 5 | TPPV | 2004.08 |
| 8 | F/62 | OD | + | 2 | 5 | 6 | - | 2004.12 |
| 9 | M/72 | OD | + | 6 | 6 | 6 | - | 2007.06 |
| Transferred patients | ||||||||
| 10 | M/72 | OS | - | 1 | 4 | 6 | TPPV | 2000.11 |
| 11 | F/70 | OD | + | 3 | 3 | 1 | TPPV | 2007.02 |
| 12 | F/70 | OD | ND | 3 | 2 | 3 | - | 2003.12 |
| 13 | F/78 | OS | ND | 24 | 3 | 5 | - | 2004.12 |
| 14 | F/70 | OS | + | 12 | 3 | 6 | TPPV | 2002.03 |
| 15 | M/63 | OD | ND | 4 | 3 | 3 | - | 2003.12 |
| 16 | F/79 | OD | ND | 1 | 5 | 3 | TPPV | 2002.02 |
| 17 | M/47 | OS | - | 1 | 3 | 5 | TPPV | 2003.08 |
| 18 | M/42 | OD | - | 1 | 6 | 6 | TPPV | 2000.11 |
| 19 | F/76 | OD | ND | 20 | 5 | 5 | TPPV | 2000.01 |
| 20 | M/47 | OS | ND | 90 | 4 | 6 | - | 2004.07 |
| 21 | M/69 | OD | - | 9 | 2 | 3 | TPPV | 2004.07 |
| 22 | M/71 | OS | - | 9 | 5 | 6 | TPPV | 2002.09 |
| 23 | F/76 | OD | ND | 7 | 5 | 5 | - | 2002.07 |
| 24 | F/69 | OD | ND | 5 | 5 | 3 | - | 2005.11 |
| 25 | F/82 | OD | - | 4 | 3 | 6 | - | 2005.04 |
| 26 | M/73 | OS | + | 2 | 1 | 1 | - | 2006.11 |
| 27 | F/74 | OD | - | 2 | 2 | 1 | TPPV | 2007.05 |
| 28 | F/72 | OD | + | 5 | 3 | 4 | TPPV | 2003.01 |
| 29 | M/66 | OD | - | 6 | 3 | 5 | TPPV | 2005.01 |
Table 2.
Microbiologic spectrum between two periods
Table 3.
Antibiotics susceptibility between two periods
| Period 1 | Period 2 | P value | |
|---|---|---|---|
| Vancomycin | |||
| Sensitive | 2 | 7 | |
| Resistant | 0 | 0 | |
| Tobramycin | |||
| Sensitive | ND* | 5 | |
| Resistant | ND | 2 | |
| TMP-SMX | 0.625 | ||
| Sensitive | 1 | 4 | |
| Resistant | 0 | 3 | |
| Ciprofloxacin | 0.583 | ||
| Senstive | 1 | 2 | |
| Resistant | 1 | 5 | |
| Ofloxacin | |||
| Sensitive | ND | 5 | |
| Resistant | ND | 2 | |
| Oxacillin | 0.583 | ||
| Sensitive | 1 | 2 | |
| Resistant | 1 | 5 |
Table 4.
Comparison of visual acu uity prognosis & risk factors betw ween two periods
| Period 1 | Period 2 | P value | |
|---|---|---|---|
| Initial VA | 0.105 | ||
| A group | 5 | 11 | |
| B group | 8 | 5 | |
| Final VA | 0.336 | ||
| A group | 3 | 6 | |
| B group | 10 | 10 | |
| Increased VA | 10 | 11 | |
| Decreased VA | 3 | 4 | |
| E group* | 3 | 6 | |
| F group† | 1 | 1 | |
| E group / Increased VA | 3/10 (30%) | 6/11 (60%) | 0.245 |
| F group / Decreased VA | 1/3 (33.3%) | 1/4 (25%) | 0.714 |
| Age | 66.92 | 69.94 | |
| Total Patients | 13 | 16 | |
| M:F | 7:6 (1.17:1) | 4:12 (1:3) | 0.114 |
| Mean Duration | 5.0 | 4.43 | |
| Total vitrectomy | 9 | 7 | 0.160 |
| Interval (day) | 6.1 | 12.14 | |
| Delayed onset type | 1 | 2 | 0.580 |
Table 5.
Comparison of risk factors between two groups
| C* Group (21) | D† Group (8) | P-value | |
|---|---|---|---|
| Mean Age | 68.0 | 68.88 | |
| M:F | 10:11 (1:1.1) | 3:5 (1:1.67) | 0.474 |
| Duration | 5.053 | 3.75 | 0.353 |
| Culture positive | 6 | 3 | 0.483 |
| DM, HTN | 11 | 4 | 0.617 |
| Total vitrectomy | 12 | 5 | 0.568 |
| Interval (day) | 9.91 | 4.50 | 0.259 |
| Delayed onset type | 3 | 0 | 0.379 |
| Initial VA (>4)‡ | 10 | 2 | 0.250 |
Table 6.
Comparison of risk factors between two groups
| E Group (9)* | D Group (8) | P-value | |
|---|---|---|---|
| Mean Age | 69.67 | 68.88 | |
| M:F | 3:6 (1:2) | 3:5 (1:1.67) | 0.627 |
| Duration | 5.13 | 3.75 | 0.391 |
| Culture positive | 3 | 3 | 0.627 |
| DM, HTN | 5 | 4 | 0.601 |
| Total vitrectomy | 5 | 5 | 0.581 |
| Interval (day) | 5.0 | 4.50 | 0.603 |
| Delayed onset type | 1 | 0 | 0.529 |
| Initial VA (>3)† | 9 | 6 | 0.206 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download