Abstract
Methods
Twenty-nine myopic eyes of 29 patients underwent 2 consecutive tests with the Humphrey Matrix 30-2 threshold program. The first and second tests were performed with and without correction for myopia. Differences between the mean deviation (Δ MD) and the pattern standard deviation (Δ PSD) were calculated, and a correlation between the spherical equivalent (SE) and Δ MD and Δ PSD was investigated. The influence of optical defocus according to the visual field (central and peripheral) and the severity of glaucomatous visual field damage (area with total deviation plot of ‘P<0.1%’ or ‘P<0.5%’ and the other area) were also evaluated.
Results
The correlation between SE and Δ MD was significant (p=0.02, r=0.62). However, the correlation between SE and Δ PSD was not significant (p=0.81, r=0.15). The differences in the mean total deviation printout value of the central and peripheral visual field groups between the two tests were 5.85±4.26dB and 5.66±3.56dB, respectively (P=0.86). The differences in the mean total deviation printout value of severely damaged and less damaged areas between the two tests were 3.16±3.39dB and 6.62±4.62dB, respectively (P<0.01).
References
1. Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989; 107:453–64.

2. Kerrigan-Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000; 41:741–8.
3. Johnson CA, Adams AJ, Casson EJ, et al. Blue-on-yellow perimetry can predict the development of glaucomatous visual field loss. Arch Ophthalmol. 1993; 111:645–50.

4. Johnson ca, Adams AJ, Casson EJ, Brandt JD. Progression of early glaucomatous visual field loss as detected by blue-on-yellow and standard white-on-white automated perimetry. Arch Ophthalmol. 1993; 111:651–6.

5. Wall M, Ketoff KM. Random dot motion perimetry in patients with glaucoma and in normal subjects. Am J Ophthalmol. 1995; 120:587–96.

6. Bosworth CF, Sample SA, Gupta N, et al. Motion automated perimetry identifies early glaucomatous field defects. Arch Ophthalmol. 1998; 116:1153–8.

7. Glovinsky Y, Quigley HA, Pease ME. Foveal ganglion cell loss in size dependent on experimental glaucoma. Invest Ophthalmol Vis Sci. 1991; 32:484–91.
8. Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988; 95:357–63.

10. Johnson CA, Samuels SJ. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 1997; 38:413–25.
11. Quigley HA. Identification of glaucoma-related visual field abnormality with the screening protocol of frequency doubling technology. Am J Ophthalmol. 1998; 125:819–29.
12. Brusini P, Salvetat ML, Zeppieri M, Parisi L. Frequency doubling technology perimetry with the Humphrey Matrix 30-2 test. J Glaucoma. 2006; 15:77–83.

13. Ko BS, Kim CY, Hong YJ. Correlation between optic disc tomography and frequency doubling technology in glaucoma suspect. J Korean Ophthalmol Soc. 2003; 44:2040–6.
14. Anderson AJ, Johnson CA. Frequency-doubling technology perimetry and optical defocus. Invest Ophthalmol Vis Sci. 2003; 44:4147–52.

15. Artes PH, Nicolela MT, McCormick TA, et al. Effects of blur and repeated testing on sensitivity estimates with frequency doubling perimetry. Invest Ophthalmol Vis Sci. 2003; 44:646–52.

16. Ito A, Kawabata H, Fujimoto N, Adachi-Usami E. Effect of myopia on frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 2001; 42:1107–10.
17. Heeg GP, Stoutenbeek R, Jansonius NM. Strategies for improving the diagnostic specificity of the frequency doubling perimeter. Acta Ophthalmol Scand. 2005; 83:53–6.

18. Heeg GP, Ponsioen TL, Jansonius NM. Learning effect, normal range, and test-retest variability of Frequency Doubling Perimetry as a function of age, perimetric experience, and the presence or absence of glaucoma. Ophthalmic Physiol Opt. 2003; 23:535–40.

19. Joson PJ, Kamantigue ME, Chen PP. Learning effects among perimetric novices in frequency doubling technology perimetry. Ophthalmology. 2002; 109:757–60.

20. Iester M, Capris P, Pandolfo A, et al. Learning effect, short-term fluctuation, and long-term fluctuation in frequency doubling technique. Am J Ophthalmol. 2000; 130:160–4.

21. Brush MB, Chen PP. Test-retest variability in glaucoma patients tested with C-20-1 screening-mode frequency doubling technology perimetry. J Glaucoma. 2004; 13:273–7.

22. Adams CW, Bullimore MA, Wall M, et al. Normal aging effects for frequency doubling technology perimetry. Optom Vis Sci. 1999; 76:582–7.

23. Legge GE, Mullen KT, Woo GC, Campbell FW. Tolerance to visual defocus. J Opt Soc Am A. 1987; 4:851–63.

24. Brusini P, Salvetat ML, Zeppieri M, Parisi L. Frequency doubling technology perimetry with the Humphrey Matrix 30-2 test. J Glaucoma. 2006; 15:77–83.

25. Kogure S, Toda Y, Crabb D, et al. Agreement between frequency doubling perimetry and static perimetry in eyes with high tension glaucoma and normal tension glaucoma. Br J Ophthalmol. 2003; 87:604–8.

Figure 1.
Correlations between SE and Δ MD (A: simple linear regression, p=0.02, r=0.62), SE and Δ PSD (B: simple linear regression, p=0.93, r=0.15) (SE: spherical equivalent, Δ MD: differences of mean deviation between the first and the second tests;Δ PSD: differences of pattern standard deviation between the first and the second tests.).
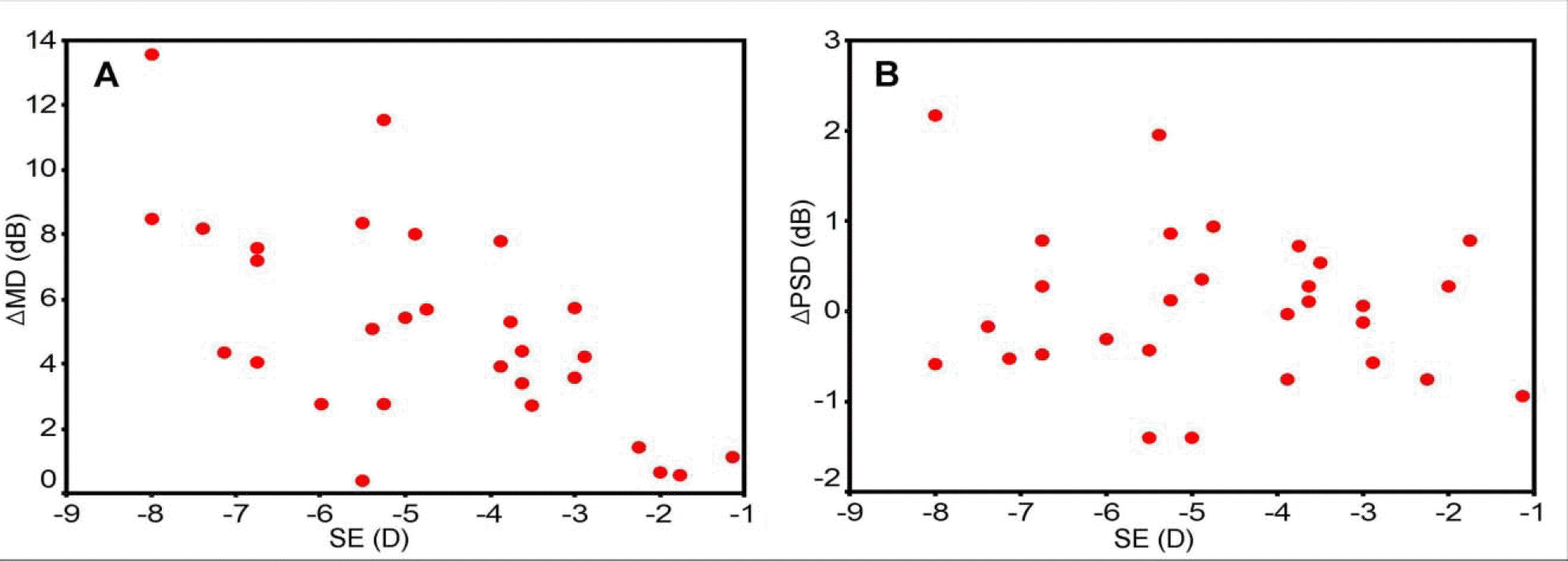
Table 1.
Difference of MD and PSD between the first (with correction) and the second (without correction) tests (MD=mean deviation; PSD=pattern standard deviation)
| The first test (with correction) | The second test (without correction) | P-value | |
|---|---|---|---|
| MD (dB) | -3.16±3.62* | -8.27±4.74* | <0.01† |
| PSD (dB) | 4.53±1.98* | 4.49±1.64* | 0.93† |
Table 2.
Changes of glaucoma hemifield test results between the first (with correction) and the second (without correction) tests
Table 3.
Differences of mean pattern standard printout values between central (within 15 degree from the fixation point) and peripheral visual field (A) and between severely damaged and less damaged visual field (B)
| Visual field (VF) | Value of total deviation printout (dB, Mean±SD) | P-value | |
|---|---|---|---|
| A | Central VF | 5.85±4.26 | 0.86* |
| Peripheral VF | 5.66±3.56 | ||
| B | Severely damaged | 3.16±3.39 | <0.01* |
| VF Less damaged VF | 6.62±4.52 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download