Abstract
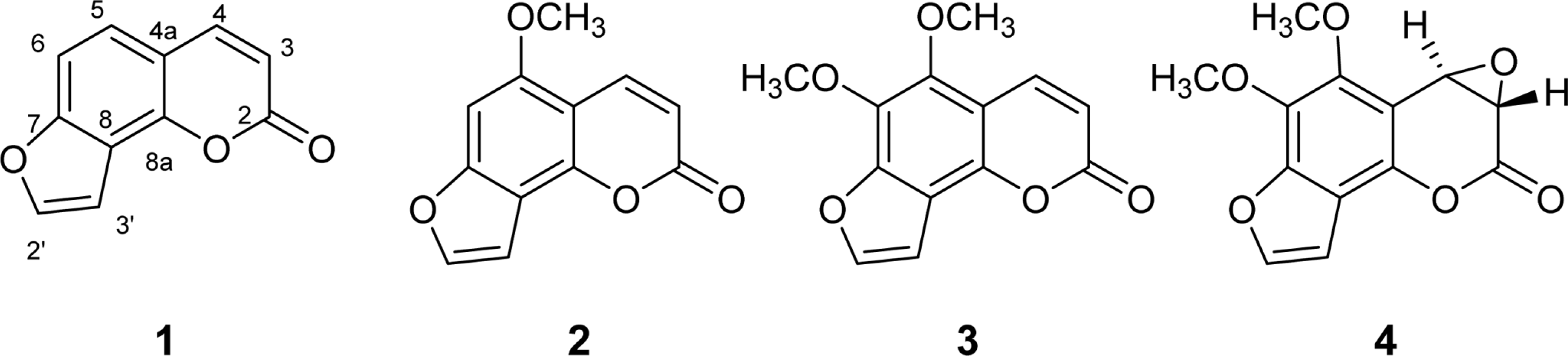
Activity-guided isolation of Heracleum moellendorffii roots led to four coumarin derivatives as acetylcholinesterase inhibitors. The structures of these isolates were characterized by spectroscopic method to be angelicin (1), isobergapten (2), pimpinellin (3), and (3S, 4R)-3, 4-epoxypimpinellin (4). All the isolated compounds 1, 2, 3, and 4 showed moderate inhibition activities against acetylcholinesterase with the IC50 values of 10.2, 18.1, 21.5 and 22.9 µM, respectively. (3S, 4R)-3, 4-Epoxypimpinellin (4) was newly isolated from the plant source.
References
(1). Lee W. T.Standard illustrations of Korean plants; Academy Pub. Co.: Seoul,. 1996. , p. 254.
(2). Zhong H. B. C.Editorial Committee of Zhong Hua Ben Cao of State Administration of Traditional Chinese Medicine of People's Republic of China Vol. 5; Shanghai Science and Technology Press; Shanghai,. 1999. 960–962.
(3). Kwon Y. S.., Cho H. Y.., Kim C. M.Yakhak Hoeji. 2000. 44:521–527.
(4). Bae D. S.., Kim C. Y.., Lee J. K.Int. Immunopharmacol. 2012. 14:734–739.
(5). Park H. J.., Nugroho A.., Jung B.., Won Y. H.., Jung Y. J.., Kim W. B.., Choi J. S.Koran J. Plant Res. 2010. 23:393–398.
(6). Nakano Y.., Matsuaga H.., Saita T.., Mori, M.: Katano M.., Okabe H.Biol. Pharm. Bull. 1998. 21:257–261.
(7). Li W.., Chen L.., Wu C.., Xin J.Asian J. Chem. 2013. 25:4701–4702.
(8). Orhan I. E.., Tosun F.., Skalicka-Wo niak K. S. J.Serb. Chem. Soc. 2016. 81:357–368. . ê z í.
(9). Dincel D.., Hatipo lu S. D.., Gören A. C.., Topcu G.Turk. J. Chem. g. 2013. 37:675–683.
(11). Ellman G. L.., Courtney K. D.., Andres Jr. V.., Feather-stone R. M.Biochem. Pharmacol. 1961. 7:88–95.
(12). Liu S.., Zhang W.., He G.., Lei P.., Li X.., Liang Y.Zhongguo Zhongyao Zazhi. 2009. 34:571–573.
(13). Steck W.., Mazurek M.Lloydia. 1972. 35:418–439.
(14). Luz R. F.., Vieira I. J. C.., Braz-Filho R.., Moreira V. F.Am. J. Analyt. Chem. 2015. 6:851–866.
(15). De Angelis G. G.., Wildman W. C.Tetrahedron. 1969. 25:5099–5112.
(16). Dhooghe L.., Maregesi S.., Mincheva I.., Ferreira D.., Marais J. P.., Lemière F.., Matheeussen A.., Cos P.., Maes L.., Vlietinck A.., Aperes S.., Pieters L.Phytochemistry. 2010. 71:785–791.
Table 1.
13C-NMR data of 1 – 4 (150 MHz, CDCl3)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download