Abstract
In the field of orofacial surgery, a red blood cell transfusion (RBCT) is occasionally required during double jaw and oral cancer surgery. However, the question remains whether the effect of RBCT during the perioperative period is beneficial or harmful. The answer to this question remains challenging. In the field of orofacial surgery, transfusion is performed for the purpose of oxygen transfer to hypoxic tissues and plasma volume expansion when there is bleeding. However, there are various risks, such as infectious complications (viral and bacterial), transfusion-related acute lung injury, ABO and non-ABO associated hemolytic transfusion reactions, febrile non-hemolytic transfusion reactions, transfusion associated graft-versus-host disease, transfusion associated circulatory overload, and hypersensitivity transfusion reaction including anaphylaxis and transfusion-related immune-modulation. Many studies and guidelines have suggested RBCT is considered when hemoglobin levels recorded are 7 g/dL for general patients and 8-9 g/dL for patients with cardiovascular disease or hemodynamically unstable patients. However, RBCT is occasionally an essential treatment during surgeries and it is often required in emergency cases. We need to comprehensively consider postoperative bleeding, different clinical situations, the level of intra- and postoperative patient monitoring, and various problems that may arise from a transfusion, in the perspective of patient safety. Since orofacial surgery has an especially high risk of bleeding due to the complex structures involved and the extensive vascular distribution, measures to prevent bleeding should be taken and the conditions for a transfusion should be optimized and appropriate in order to promote patient safety.
In the field of orofacial surgery, a red blood cell transfusion (RBCT) is often needed during either double jaw or oral cancer surgery. RBCT can be a life-saving procedure for most patients with acute anemia caused by perioperative bleeding; on the contrary, it also presents various risks, such as infectious complications (viral and bacterial), transfusion-related acute lung injury, ABO and non-ABO associated hemolytic transfusion reactions, febrile non-hemolytic transfusion reactions, transfusion associated graft-versus-host disease, transfusion-associated circulatory overload, and hypersensitivity transfusion reactions, including anaphylaxis, and transfusion-related immune-modulation (TRIM) [12]. In the field of transfusion medicine, three studies have used propensity score matching with regard to transfusion and mortality namely, the ABC [3], CRIT [4], and SOAP [5] studies. The ABC and CRIT studies considered RBCT as an independent risk factor for mortality, whereas the SOAP study proved that RBCT increased the survival rate [345]. Although these results seem to contradict one another, the differences were attributed to the influence of introducing leukocyte-depleted RBCs in the SOAP study. Moreover, there are other studies involving prospective, adequately powered, randomized controlled trials that have applied restrictive and liberal transfusion strategies with respect to mortality, and more recently, a greater emphasis on restrictive strategy [678].
As shown, the research in this area remains ongoing and can be quite controversial. Accordingly, the present review aimed to summarize transfusion based on the studies that have been previously completed on this topic to date and to discuss the role of perioperative RBCT in the field of orofacial surgery.
In 1628, William Harvey, a British physician and physiologist, first presented the theory of blood circulation. While in 1665, Richard Lower, another British physician, first reported on transfusion between animals at the Royal Society. In 1667, Jean-Baptiste Denys, a French physician, transfused the blood of sheep to a 15-year-old boy for the first time and the boy survived. In 1818, James Blundell, a British obstetrician, became the first to transfuse human blood to a patient with postpartum hemorrhage. In 1840, Samuel Armstrong Lane at St George's Hospital Medical School in London used whole blood transfusion for the first time to treat hemophilia. Then in 1900, an Austrian physician and immunologist Karl Landsteiner differentiated blood agglutinins and divided them into ABO groups, and subsequently, RBC transfusions have been performed for over 100 years as a treatment modality for moderate-to-severe anemia [9].
Oxygen transfer to the tissues in the human body involves RBCs that carry hemoglobin-bound oxygen from the lungs to the cells. Under resting conditions, the amount of oxygen supplied exceeds the oxygen demand of the tissues. This margin of safety for oxygen transport is needed for emergency situations, such as bleeding, when oxygen demand spikes rapidly; however, if excessive bleeding exceeds this safety margin, transfusion becomes necessary in order to supply enough oxygen to the peripheral vascular system and the tissues.
RBCT increases the ability to deliver oxygen to hypoxic tissues under acute anemia conditions (perioperative bleeding), as well as with diseases such as sickle cell disease or malaria. It is also responsible for carrying out a hemostatic role by increasing coagulation [10], and recent findings have indicated its involvement in clot contractions [11]. Moreover, it is also performed for the purpose of plasma volume expansion in cases of possible clinically significant perioperative bleeding.
The indications and triggers for RBCT are on-going issues. There have been many studies and there are still on-going studies in search of an answer. Based on studies to date, there are two strategies. Historically, the threshold that triggered a RBCT has been based on a liberal strategy (hemoglobin [Hb] level below 10 g/dL or hematocrit [Hct] below 30%). In 1988, the “10/30 Rule” was presented at the National Institutes of Health Consensus Development Conference, which presented the level of RBCT during perioperative period to be less than Hb 10 g/dL and Hct 30% and transfusions were performed based on those values for a while [12]. In 1999, a randomized, multicenter, controlled clinical trial that compared a restrictive transfusion trigger (Hb 7 g/dL) and liberal transfusion trigger (Hg 10 g/dL) in critically ill patients found that the mortality rate was lower in the group with the restrictive transfusion strategy than that of the group with the liberal transfusion strategy (22.3 % vs 28.1 %, P = 0.05) [613]. Recently, the restrictive strategy (Hb level below 7 g/dL) has become more accepted due to the accumulation of evidence regarding the negative impact on prognoses following RBCT per the liberal strategy as well as the complications and costs associated with RBCT [1415161718192021].
Recent reports on the guidelines for the RBCT threshold are summarized in Table 1. The American Society of Anesthesiologists sets the RBCT threshold as Hb < 6 g/dL, and for Hb between 6-10 g/dL, the decision is left up to the discretion of the anesthesiologist [222324], while the Society of Cardiovascular Anesthesiologists and several other associations has set the transfusion threshold at Hb < 7 g/dL [1325262728293031]. The European Society of Anesthesiology recommends maintaining Hb between 7-9 g/dL, regardless of perioperative bleeding [32]. The American Academy of Family Physicians has set the transfusion trigger at Hb between 7-9 g/dL for immature infants or cases involving cyanotic heart disease, severe hypoxemia, active blood loss, or hemodynamic instability [13]. The Italian Society of Transfusion Medicine and Immunohematology has set the transfusion trigger at Hb between 6-10 g/dL for cases involving coronary artery disease or cardiovascular disease [24]. The European Society of Cardiology has set the transfusion trigger at Hb of 7 g/dL when the acute coronary syndrome is present [33] and the American College of Physicians has set it at Hb between 7-8 g/dL when coronary heart disease is present [34]. Meanwhile, the UK National Clinical Guideline Centre and American Association of Blood Banks have set the transfusion trigger at Hb of 8 g/dL for cases involving cardiovascular diseases [2935].
Therefore, based on studies and guidelines up to now, it would be appropriate to set the RBC transfusion trigger at Hb of 7 g/dL, while applying the higher threshold of 8-9 g/dL for patients with cardiovascular disease or hemodynamically unstable patients. Due to recent studies, an additional issue to consider when performing RBCT has been added. Continuous noninvasive Hb (SpHb) monitoring can be helpful for determining the RBCT trigger [3637]. Another study reported that by checking the trend of the change in Hb by continuously monitoring SpHb, the amount of bleeding can be predicted and allow for a rapid response to bleeding. It is also helpful in double-jaw surgery [38].
Although there is a clear-cut therapeutic benefit of relieving hypoxia in tissues with a RBCT, it should be performed after careful evaluation of its risks and benefits. A meta-analysis that analyzed 45 studies on the effects of RBCT on prognoses found that RBCT had a negative impact on prognoses, while two studies showed a neutral risk and one study placed more emphasis on its benefits [39]. The following are the details of each risk.
Interest in transfusion-transmitted infections peaked in the 1980s due to transmission of human immunodeficiency virus (HIV) and hepatitis C virus [40]. Subsequent medical advances, along with the use of serological and nucleic acid testing assays, have led to a drastic decline in the number of transfusion-transmitted infections, including HIV and the hepatitis viruses (hepatitis A, B, C) [4142]. With respect to infections, today is historically the safest time for a transfusion, but with the emergence of novel viruses, such as the recent Zika virus, transfusion-transmitted cytomegalovirus (CMV) still occurs and it remains a key issue for immunosuppressed patients [43]. Moreover, the post-transfusion infection rate may also increase due to TRIM [44].
A major potential consequence of RBCT is alloimmunization to blood group antigens. Alloimmunization can result in acute hemolytic transfusion reactions [45], potentially producing significant morbidity and mortality. One of the most important transfusion-related issues is still administrative error, which is associated with hemolytic transfusion reaction and ABO incompatibilities that lead to mortality in many cases.
This is a more common reaction than hemolytic transfusion reaction. It is caused by cytokines within the units of red cells. Such cytokines occur when the donor red cells break up during storage or they originate from the donor leukocytes. Such reactions can be reduced by using a leukocyte filter [46].
Currently, TRALI is one of the most dangerous transfusion-associated complication [1]. TRALI is defined as a new acute lung injury occurring within 6 h post transfusion in patients without risk factors for acute lung injury. TRALI typically manifests as shortness of breath, fever, and hypotension [47]. Antibody-medicated TRALI (immune TRALI) is now recognized as one of the most common causes of transfusion-associated major morbidity and death in the Western world [48]. Although TRALI can occur as a result of all types of blood products, it is known to occur more commonly with plasma and pooled buffy coat-derived platelet products, rather than with RBCs (fatal TRALI incidence: plasma, 1:200,000-300,000; platelets, 1:300,000-400,000; RBCs, 1:2,500,000-2,900,000) [49]. The mechanism of TRALI has not been clearly identified, but it can be explained by the 2-hit hypothesis. Antibodies, as well as alternate substances in blood products result in neutrophil activation, which, in a susceptible patient, results in TRALI [49].
Hypersensitivity transfusion reaction is a very rare reaction. The biologic mechanism associated with this remains largely unknown, but patients who have had a transfusion-associated adverse reaction may have had an underlying immunodeficiency [50]. Particularly in cases transfused with blood products containing plasma, the development of anaphylaxis, a severe form of hypersensitivity, is highly associated with IgA deficiency, which appears from the production of Ig E Ab against Ig A [51].
Although it is called transfusion-associated circulatory overload (TACO), it is not easy to differentiate between TACO and TRALI. It is a rare complication with less than 100 articles published since its introduction during the 1990s [52]. Therefore, it is difficult to determine whether the exacerbation of pulmonary function occurred as an immunomodulation effect or due to circulatory overload [53]. Other assessments of volume overload, such as jugular venous pressure and B-natriuretic peptide (BNP) levels, can help distinguish the two [46].
Transfusion may trigger an immunologic reaction from the recipient antibody coming together with the donor antigen. This immunologic reaction that has a negative effect on the human immune system is referred to as “transfusion related immunomodulation (TRIM)” [54]. TRIM is a complex physiological reaction, which is mediated by residual leukocytes, apoptotic cells, and numerous biological response modifiers such as cytokines, soluble mediators, and soluble HLA peptides [55]. TRIM can be explained by two mechanisms, the first of which is HLA-dependent and directed against adaptive immunity, while the second is mild, non-specific, and directed against innate immunity [56]. Such mechanisms are related to the release of immunosuppressive prostaglandins, suppression of cytotoxic cell and monocyte activity, inhibition of interleukin-2 (IL-2) production, and an increase in regulatory T cells (Tregs) and suppressor T-cell activity [57585960].
TRIM is associated with the following: transforming growth factor beta (TGF-β), a transfusion-related inflammatory/immunosuppressive cytokine [61]; secondary cytokines secreted by phagocytosis of apoptotic cells; and non-polar lipids and a mixture of pro-inflammatory lyso-phosphatidylcholines (lyso-PCs) contained in RBCs [62]. Lyso-PC regulates the activation of natural killer (NK) T cells and T lymphocytes [63]; induces dendritic cell maturation [64]; acts as an NK cell chemoattractant [65]; and stimulates the production of pro-inflammatory cytokines [66]. Eicosanoids such as prostaglandins, leukotrienes, and thromboxanes can accumulate in RBCs [67], and all of these inflammatory mediators may suppress immunity or aggravate tumors [68697071].
There is evidence that leukocyte-containing red blood cell concentrates have negative effects, such as increased postoperative complications, associated with mortality rate [72]. Consequently, the recent trend is to use leukoreduced RBC concentrates; as a result, the risk of febrile transfusion reactions and CMV transfusion transmissions is reduced [73]. However, even after leukoreduction, a few leukocytes and cytokines may remain [7475] and can cause the secretion of IL-6, IL-10, and tumor necrosis factor alpha (TNF-α) or activation of Tregs [7677]. The activation of Tregs is antigen non-specific; it can occur due to lipopolysaccharide (LPS) and, through the Toll-like receptor-4 pathway, lead to immune suppression [78].
Apoptotic cells, which are created while blood is being stored, activate and secrete TGF-β to trigger immunosuppression [6179]. Apoptotic cells express phosphatidylserine on the surface [80]. Phosphatidylserine not only induces secretion of anti-inflammatory cytokines, such as IL-10 and TGF-β, but it also suppresses secretion of inflammatory cytokines, such as IL-12, IL-1β, IL-6, and TNF [818283].
Immunomodulation may be helpful at times, as indicated by a study result that showed a transfusion improved the survival of the kidney graft in patients who received one [84]. Moreover, among Crohn's disease patients with small bowel resection, the recurrence rate of Crohn's disease was lower in the group that received a transfusion when compared to the group that did not [85]. Therefore, when immunosuppression is required for therapeutic purposes, immunomodulation from a transfusion may be helpful.
The American Society of Clinical Oncology/American Society of Hematology introduced transfusion as an effective treatment strategy for improving the health of cancer patients with chemotherapy-associated anemia [86]. However, numerous studies have reported that recurrence and metastasis of cancer increased following transfusion [87888990] and this is suspected to be the result of immunosuppression following allogenic RBCT [91]. Immunomodulation due to inflammatory mediators, residual leucocytes, and apoptotic cells is associated with recurrence and progression of cancer, while platelets, microparticles, and FFP are known to stimulate growth and spreading of the tumor [929394].
TGF-β suppresses NK cell activity and induces Tregs activation, in conjunction with TNF-α [88]. Moreover, in a tumor microenvironment, TGF-β contributes to the osteoclastic degeneration of the bone matrix necessary for the establishment of bone metastasis [9596]. Inflammatory mediators, such as TNF-β and lyso-PCs regulate the activation of NK cells and T cells, while also promoting the production of pro-inflammatory cytokines. [6366]. The aforementioned inflammatory mediators, residual leukocytes, and apoptotic cells induce immunomodulation by various mechanisms, which can directly or indirectly contribute to tumor growth.
While only a few studies have reported that RBCT in colorectal cancer (CRC) cases did not have a negative effect on overall mortality, 5-year survival, recurrence, metastasis, complications, and prognosis [979899100], many studies have reported the opposite [90101102103104105106107108]. In fact, there are even reports of perioperative RBCT in CRC cases causing increased rate of infection [109110]. Even in cases that required liver resection due to metastasis of CRC to the liver, RBCT had a negative effect on free survival, recurrence, and prognosis [111112]. RBCT had a negative effect on clinical outcomes, mortality, recurrence, and complications in cases that required liver resection due to primary cancer in the liver [113114115]. It also had a negative effect on mortality, recurrence, and prognosis in cases that required surgery for gastric cancer [116117118]. Moreover, it was also reported that RBCT had a negative effect on long-term survival, recurrence, and long-term outcome in cases involving surgery for esophageal cancer [119120121122], while allogenic RBCT in cervical or ovarian cancer cases resulted in decreased survival and poor prognosis from increased metastasis [89123124]. When RBCT was performed during radical cystectomy for bladder cancer, it caused the postoperative tumor recurrence and cancer-specific mortality to increase [125]. When RBCT was performed on patients undergoing surgery for lung cancer, RBCT had a negative effect on 5-year disease-free survival [126].
Many cases of cancer are encountered in the field of orofacial surgery, which occasionally require perioperative RBCT. There have been many reports indicating that using RBCT during oral cancer surgery resulted in adverse outcomes. In a study comprised of 520 patients who underwent surgery for oral and oropharyngeal cancer, those who received an allogenic RBCT showed increased postoperative complications, which was reported to be associated with the amount of transfusion received [127]. In another study comprised of 276 patients who underwent oral cancer surgery, RBCT was reported to increase the rate of surgical-site infection [128]. A retrospective study on 1,693 patients who underwent oral cavity cancer surgery reported that RBCT was a risk factor for wound infection [129]. In various studies on patients who underwent surgery for oral cavity squamous cell carcinoma, performing RBCT with 3 units (U) or more shortened the survival and had a negative effect on prognosis [130131]. Moreover, RBCT during surgery for head and neck cancer resulted in an increased recurrence and decreased survival [132].
Double-jaw surgery can potentially cause vascular injury or lead to a prolonged surgery, which may result in severe blood loss. Some studies have reported up to 1191 mL [133] of blood loss requiring fluid replacement with crystalloids, colloids, or blood products. Studies on the effects of RBCT during double-jaw surgery and prognosis are lacking. However, adverse effects from other surgeries may be anticipated. Therefore, induced/controlled hypotension may be considered as a method for reducing perioperative bleeding and RBCT [134135]. Study results on patients who underwent a Le Fort I osteotomy showed that the average blood loss in a group that used hypotensive anesthesia was 740 ml, whereas the average blood loss in the control group was 400 ml [136]. Furthermore, preoperative and intraoperative administration of the antifibrinolytic agent, tranexamic acid, is effective for controlling blood loss and improving the quality of the surgical field [137].
There are numerous study results indicating that RBCT in non-cancer surgeries result in poor prognosis. RBCT of 3 or more U during liver transplantation surgery increased the length of the hospital stay [138]. The patient group that received RBCT during a coronary artery bypass had a 5-year mortality that was twice as high as that of the group without RBCT [139]. In transcatheter aortic valve implantation surgery cases, using RBCT resulted in an increased 12-month mortality and prolonged hospital stay [140]. Even in major vascular surgery cases, RBCT increased the risk of myocardial infarction and 30-day mortality [141]. RBCT also induced a minor wound-healing disturbance in hip replacement surgery cases, while also increasing the length of the hospital stay [142]. For general surgery, 1 U RBCT increased the risk of 30-day mortality, composite morbidity, pneumonia, and sepsis/shock, while 2 U RBCT increased these outcome risks, as well as surgical-site infections [143].
Are there differences in adverse effects according to the amount of transfused RBCs? A study comprised of 89,000 patients reported that the mortality and morbidity were higher in the patient group that received 1U RBCT than the non-transfused patient group, showing a dose-dependent tendency [144]. Besides mortality, wound problems, pulmonary complications, postop renal dysfunction, sepsis, morbidity, and length of the hospital stay were reported to be high in the group that received RBCT than that in the group that did not and caution was recommended when performing transfusions on patients with a mild anemia [144]. Other studies have reported poor outcomes when 2 U of RBC was used compared to that when 1 U was used [143]. Even in the field of orofacial surgery, there is evidence that a greater amount of RBCT has a greater negative effect on the outcome [127130]. In contrast, a study that compared restrictive (Hb < 7 g/dL) vs liberal (Hb < 9 g/dL) strategy on ICU patients who underwent major cancer surgery reported a higher rate of major complications in the restrictive group despite using a lesser amount of RBCT [145].
Orofacial surgery may be associated with significant bleeding. The amount of blood loss and transfusion is always a concern during double jaw surgery. In single jaw surgery, RBCT is considered unnecessary [146-148]. However, studies published prior to 2005 show that the average amount of bleeding in double jaw surgery ranged from 344 ml to 889 ml, while the percentage of RBCT varied widely between 0.8 % and 30 % [146148149150151152]. Meanwhile, recent studies (Table 2), including that by Tang et al., report that the mean blood loss was 420.3 ± 175.9 ml (range 183.0-965.6 ml) and there were no cases that required RBCT [153]. Ervens et al. report that the mean blood loss under normotensive anesthesia was 1,021.6 ml (range 300-2600 ml), while the preoperative Hb was 13.5 g/dL (range 11.6-16.0 g/dL) and the postoperative Hb was 9.3 g/dL (range 6.4-12.8 g/dL) [133]. Varol et al. reported a mean blood loss of 377.0 ± 111.2 mL (range 180-625 mL), preoperative Hb of 14.0 ± 1.9 g/dL (range 10.3-17.2 g/dL), and postoperative Hb of 11.8 ± 2.0 g/dL (range 8.2-16.2 g/dL) [135]. Sankar et al. reported a mean blood loss of 278.3 ± 78.2 mL [137]. Faverani et al. reported a mean blood loss of 453.3 ± 168.5 mL, preoperative Hb of 14.5 ± 1.2 g/dL, and postoperative Hb of 12.4 ± 1.2 g/dL and that RBCT was performed on 2 out of 15 patients [154]. As shown, there are differences in the measured amount of blood loss between studies. While recently published studies have shown that a few cases required RBCT, the amount of blood loss in double-jaw surgery was higher than that during other surgeries and some cases required transfusion. Therefore, it is necessary to prepare for situations that require a transfusion while continuously monitoring perioperative bleeding.
RBC concentrates are produced by removing the plasma fraction from centrifuged whole blood. Using 1 U of RBCs can typically increase the hemoglobin level by about 1 g/dL [155]. The number of storage days of RBC concentrates produced in this manner increased from 21 days to 42 days (at 1-6 ℃) when they were treated with hypotonic solutions such as anticoagulant-preservative solution, acid citrate dextrose, citrate phosphate dextrose, and citrate phosphate dextroseadenine [156157]. Based on this development, the study was conducted by dividing the RBC concentrates into groups of “young (fresh)” (< 14-21 days) and “old” (> 21 days) RBC concentrates. The increased storage duration of RBCs in hypotonic solution can lead to hemolysis, morphological changes, lactic acid accumulation, increased potassium/calcium, decreased 2,3-DPG and ATP, decreased pH and glycolysis rate, and the accumulation of various residues [158159160]. Because of this reason, transfusion using “old” RBC may cause adverse effects, including a higher infection risk and higher transfusion-dependent mortality rate [161162163]. Actually, a large-scale study conducted in Australia, Canada, Israel, and the US showed that the group that received a transfusion of “young” RBCs had a better prognosis than the group that was transfused with “old” RBCs [164]. However, there are other studies that report no difference in prognoses between the two groups [165] and a study that reports that an “old” RBC transfusion had the benefit of reducing cancer recurrence [163]. Therefore, this is an issue still under debate and there is the need for a sufficient discussion on issues that can arise a preference for “young” RBCs.
Various aforementioned RBCT risks are mostly associated with allogenic transfusion. An allogenic RBCT is not only the cause of various adverse events, but it also increases the economic and psychological burden of the patient. On the other hand, an autologous transfusion can avoid various adverse events associated with an allogenic transfusion, while addressing the issue of lack of blood and reducing the economic burden of the patient [166167]. A randomized study on CRC surgery reported that the recurrence rate was lower with autologous RBCT compared to that of an allogenic RBCT [168]. An autologous RBCT is frequently used during double-jaw surgery in the field of orofacial surgery and an autologous RBCT can serve as a method for reducing allogenic RBCT [169]. The following are the three options for an autologous RBCT: preoperative autologous blood donation (PABD), acute normovolemic hemodilution (ANH), and intraoperative and postoperative autotransfusion.
PABD refers to the method of drawing blood from the patient preoperatively and keeping it until reinjecting it into the patient intra- or postoperatively. Repeated blood donations before surgery can stimulate bone marrow cell proliferation, stimulate erythrocyte regeneration, increase hematopoietic function in patients after surgery, accelerate the patient's hematopoietic recovery after surgery, is conducive for wound healing, and reduces the chances of infection caused by immunoreaction from allogenic RBCT [170]. In addition, the improved autologous RBCT has a number of advantages such as the mild dilution of blood, improved microcirculation, reduction in blood viscosity, and prevention of hypoxia caused by anemia after blood donation [171].
For Hb ≥ 11 g/dL, donation 4 days prior to surgery is recommended, but donations for a ≥ 8-9 ml/kg is not recommended. Oral iron may be helpful for restoring the Hb to the level prior to donation. Contraindications include bacteremia and acute localized infection, myocardial infarction in the past 6 months, unstable angina, aortic stenosis, congestive heart failure, significant ventricular arrhythmias, marked uncontrolled hypertension, and cerebrovascular accident within 6 months.
ANH is a method of autologous transfusion, which was first introduced in 1946, and is still widely used today [171]. ANH is generally performed under general anesthesia and prior to the start of surgery. Blood is drawn from the patient and stored and an equivalent amount of crystalloid (1:3) or colloid (1:1) fluid is added to maintain the plasma volume. If required intra- or postoperatively, the collected blood can be reinjected to the patient [172]. ANH can effectively reduce erythrocyte loss caused by perioperative bleeding and reduce allogenic RBCT [173174]. This method is widely used for postpartum hemorrhage and cancer and orthopedic surgeries, such as joint replacement and spine surgery. Moreover, this method is used in the field of dentistry when cancer or double jaw patient with expected bleeding is a Jehovah's Witness [175]. This method offers several advantages. The first is the reduction of perioperative erythrocyte loss since crystalloid or colloid, in equivalent amount as blood collected, is used to restore volume. This enhances the body's tolerance and reduces actual blood loss [172176]. The second advantage is that it is simpler and less costly than the PABD method. The third advantage is that it is the only method that can provide fresh autologous blood that has almost no effect on the functions of platelets and clotting factors. ANH may be used in cases where Hb is > 11 g/dL and platelets are > 100 × 109 /L and the prothrombin time and cardio-pulmonary function are normal [177].
Intraoperative or postoperative autotransfusion refers to a method of transfusion in which the blood in the body cavity of a patient, blood lost during surgery, and postoperatively drained blood, can be recovered through a blood recovery device [171]. The blood subsequently undergoes anticoagulation, filtration, and washing and is finally transfused back to the patient [178]. The American Association of Blood Banks guidelines recommended intra- or postoperative autotransfusion when perioperative bleeding (more than 20% total volume) is expected. However, it is not recommended for intraoral surgeries, such as oral cancer or double jaw surgeries, due to the risk of systemic infection caused by the resident bacteria inside the oral cavity.
The answer to the question whether RBCT during perioperative period is beneficial or harmful to patient is not an easy one. In the field of orofacial surgery, transfusion is performed for the purpose of oxygen transfer to hypoxic tissues and plasma volume expansion when there is bleeding. Many studies and guidelines have suggested a hemoglobin level of 7 g/dL for general patients and 8-9 g/dL for patients with cardiovascular disease or hemodynamically unstable patients as the trigger for RBCT. However, RBCT is an essential treatment for surgeries and it is often required in emergency cases. We need to consider perioperative bleeding, various clinical situations, level of intra- and postoperative patient monitoring, and various problems that may arise from a RBCT in a comprehensive manner from the perspective of patient safety. Since orofacial surgery carries an especially high risk of bleeding due to the complex structures involved and extensive vascular distribution, measures to prevent bleeding should be taken and the conditions for a transfusion should be optimized and appropriate in order to promote patient safety.
References
1. Vamvakas EC, Blajchman MA. Transfusion-related mortality: The ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009; 113:3406–3417. PMID: 19188662.

2. Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013; 381:1845–1854. PMID: 23706801.

3. Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002; 288:1499–1507. PMID: 12243637.

4. Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The crit study: Anemia and blood transfusion in the critically ill--current clinical practice in the united states. Crit Care Med. 2004; 32:39–52. PMID: 14707558.

5. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in european intensive care units: Results of the soap study. Crit Care Med. 2006; 34:344–353. PMID: 16424713.

6. Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, canadian critical care trials group. N Engl J Med. 1999; 340:409–417. PMID: 9971864.
7. Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The premature infants in need of transfusion (pint) study: A randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006; 149:301–307. PMID: 16939737.

8. Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: The tracs randomized controlled trial. JAMA. 2010; 304:1559–1567. PMID: 20940381.
9. Schmidt PJ, Ness PM. Hemotherapy: From bloodletting magic to transfusion medicine. Transfusion. 2006; 46:166–168. PMID: 16441589.

10. Valeri CR, Khuri S, Ragno G. Role of the hct in the treatment of thrombocytopenic patients. Transfusion. 2003; 43:1761–1763. author reply 3. PMID: 14674376.

11. Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, et al. Clot contraction: Compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014; 123:1596–1603. PMID: 24335500.

12. Perioperative red cell transfusion. Natl Inst Health Consens Dev Conf Consens Statement. 1988; 7:1–6.
13. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: Indications and complications. Am Fam Physician. 2011; 83:719–724. PMID: 21404983.
14. Hogshire L, Carson JL. Red blood cell transfusion: What is the evidence when to transfuse? Curr Opin Hematol. 2013; 20:546–551. PMID: 23945271.
15. Liumbruno GM, Vaglio S, Biancofiore G, Marano G, Mengoli C, Franchini M. Transfusion thresholds and beyond. Blood Transfus. 2016; 14:123–125. PMID: 26950940.
16. Freedman J. Transfusion--whence and why. Transfus Apher Sci. 2014; 50:5–9. PMID: 24393629.
17. Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014; 371:1381–1391. PMID: 25270275.

18. Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013; 368:11–21. PMID: 23281973.

19. Jairath V, Kahan BC, Gray A, Dore CJ, Mora A, James MW, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (trigger): A pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015; 386:137–144. PMID: 25956718.
20. Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011; 365:2453–2462. PMID: 22168590.

21. Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care-associated infection after red blood cell transfusion: A systematic review and meta-analysis. JAMA. 2014; 311:1317–1326. PMID: 24691607.
22. Simon TL, Alverson DC, AuBuchon J, Cooper ES, DeChristopher PJ, Glenn GC, et al. Practice parameter for the use of red blood cell transfusions: Developed by the red blood cell administration practice guideline development task force of the college of american pathologists. Arch Pathol Lab Med. 1998; 122:130–138. PMID: 9499355.
23. Management ASoATFoPB. Practice guidelines for perioperative blood management: An updated report by the american society of anesthesiologists task force on perioperative blood management. Anesthesiology. 2015; 122:241–275. PMID: 25545654.
24. Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Recommendations for the transfusion management of patients in the peri-operative period. Iii. The post-operative period. Blood Transfus. 2011; 9:320–335. PMID: 21627922.
25. Napolitano LM, Kurek S, Luchette FA, Anderson GL, Bard MR, Bromberg W, et al. Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. J Trauma. 2009; 67:1439–1442. PMID: 20009700.

26. Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al. Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009; 37:3124–3157. PMID: 19773646.

27. Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011; 91:944–982. PMID: 21353044.

28. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012; 107:345–360. quiz 61. PMID: 22310222.

29. Alexander J, Cifu AS. Transfusion of red blood cells. JAMA. 2016; 316:2038–2039. PMID: 27732722.

30. Centre NCG. Acute upper gastrointestinal bleeding. (evidence update august 2014. A summary of selected new evidence relevant to nice clinical guideline 141. Evidence update 63). London: Royal College of Physicians;2012.
31. Excellence TNIfHaC. Blood transfusion nice guideline. Accessed on 31/03/2017. Available at: https://wwwniceorguk/guidance/ng24.
32. Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: Guidelines from the european society of anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017; 34:332–395. PMID: 28459785.
33. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. Esc guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: The task force for the management of acute coronary syndromes (acs) in patients presenting without persistent st-segment elevation of the european society of cardiology (esc). Eur Heart J. 2011; 32:2999–3054. PMID: 21873419.
34. Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P. Treatment of anemia in patients with heart disease: A clinical practice guideline from the american college of physicians. Ann Intern Med. 2013; 159:770–779. PMID: 24297193.

35. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical practice guidelines from the aabb: Red blood cell transfusion thresholds and storage. JAMA. 2016; 316:2025–2035. PMID: 27732721.
36. Berkow L, Rotolo S, Mirski E. Continuous noninvasive hemoglobin monitoring during complex spine surgery. Anesth Analg. 2011; 113:1396–1402. PMID: 21965372.

37. Miller RD, Ward TA, Shiboski SC, Cohen NH. A comparison of three methods of hemoglobin monitoring in patients undergoing spine surgery. Anesth Analg. 2011; 112:858–863. PMID: 21385985.

38. Kim SH, Choi JM, Kim HJ, Choi SS, Choi IC. Continuous noninvasive hemoglobin measurement is useful in patients undergoing double-jaw surgery. J Oral Maxillofac Surg. 2014; 72:1813–1819. PMID: 24813777.

39. Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008; 36:2667–2674. PMID: 18679112.

40. Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010; 50:2080–2099. PMID: 20738828.

41. Hourfar MK, Jork C, Schottstedt V, Weber-Schehl M, Brixner V, Busch MP, et al. Experience of german red cross blood donor services with nucleic acid testing: Results of screening more than 30 million blood donations for human immunodeficiency virus-1, hepatitis c virus, and hepatitis b virus. Transfusion. 2008; 48:1558–1566. PMID: 18466173.

42. Lindholm PF, Annen K, Ramsey G. Approaches to minimize infection risk in blood banking and transfusion practice. Infect Disord Drug Targets. 2011; 11:45–56. PMID: 21303341.
43. Ziemann M, Thiele T. Transfusion-transmitted cmv infection - current knowledge and future perspectives. Transfus Med. 2017; 27:238–248. PMID: 28643867.
44. Sharma AD, Slaughter TF, Clements FM, Sreeram G, Newman MF, Phillips-Bute B, et al. Association of leukocyte-depleted blood transfusions with infectious complications after cardiac surgery. Surg Infect (Larchmt). 2002; 3:127–133. PMID: 12519479.

45. Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014; 54:137–148. PMID: 23721209.

47. Webert KE, Blajchman MA. Transfusion-related acute lung injury. Transfus Med Rev. 2003; 17:252–262. PMID: 14571393.

48. Bux J. Antibody-mediated (immune) transfusion-related acute lung injury. Vox Sang. 2011; 100:122–128. PMID: 21175662.

49. Shaz BH, Stowell SR, Hillyer CD. Transfusion-related acute lung injury: From bedside to bench and back. Blood. 2011; 117:1463–1471. PMID: 20944069.

50. Adkinson NF Jr, Burks W, Busse WW, Holgate ST, Lemanske RF Jr, O'Hehir RE. Middleton's allergy principles & practice. 8th edition. Elsevier;2014. p. 1152.
51. Anani W, Triulzi D, Yazer MH, Qu L. Relative iga-deficient recipients have an increased risk of severe allergic transfusion reactions. Vox Sang. 2014; 107:389–392. PMID: 25220631.

52. Popovsky MA, Audet AM, Andrzejewski C Jr. Transfusion-associated circulatory overload in orthopedic surgery patients: A multi-institutional study. Immunohematology. 1996; 12:87–89. PMID: 15387748.

53. Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010; 137:209–220. PMID: 20051407.

54. Muszynski JA, Spinella PC, Cholette JM, Acker JP, Hall MW, Juffermans NP, et al. Transfusion-related immunomodulation: Review of the literature and implications for pediatric critical illness. Transfusion. 2017; 57:195–206. PMID: 27696473.

55. Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (trim): An update. Blood Rev. 2007; 21:327–348. PMID: 17804128.

56. Blajchman MA. The clinical benefits of the leukoreduction of blood products. J Trauma. 2006; 60:S83–S90. PMID: 16763486.

57. van Twuyver E, Mooijaart RJ, ten Berge IJ, van der Horst AR, Wilmink JM, Kast WM, et al. Pretransplantation blood transfusion revisited. N Engl J Med. 1991; 325:1210–1213. PMID: 1922208.

58. Jensen LS, Andersen AJ, Christiansen PM, Hokland P, Juhl CO, Madsen G, et al. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992; 79:513–516. PMID: 1611441.

59. Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002; 102:6–12. PMID: 11792145.

60. Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002; 16:144–160. PMID: 11941576.

61. Dzik WH. Apoptosis, tgf beta and transfusion-related immunosuppression: Biologic versus clinical effects. Transfus Apher Sci. 2003; 29:127–129. PMID: 12941349.
62. Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil nadph oxidase. J Lab Clin Med. 1994; 124:684–694. PMID: 7964126.
63. Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, et al. Recognition of lyso-phospholipids by human natural killer t lymphocytes. PLoS Biol. 2009; 7:e1000228. PMID: 19859526.

64. Coutant F, Perrin-Cocon L, Agaugue S, Delair T, Andre P, Lotteau V. Mature dendritic cell generation promoted by lysophosphatidylcholine. J Immunol. 2002; 169:1688–1695. PMID: 12165488.

65. Jin Y, Damaj BB, Maghazachi AA. Human resting cd16-, cd16+ and il-2-, il-12-, il-15- or ifn-alpha-activated natural killer cells differentially respond to sphingosylphosphorylcholine, lysophosphatidylcholine and platelet-activating factor. Eur J Immunol. 2005; 35:2699–2708. PMID: 16078278.
66. Olofsson KE, Andersson L, Nilsson J, Bjorkbacka H. Nanomolar concentrations of lysophosphatidylcholine recruit monocytes and induce pro-inflammatory cytokine production in macrophages. Biochem Biophys Res Commun. 2008; 370:348–352. PMID: 18371300.

67. Jacobi KE, Wanke C, Jacobi A, Weisbach V, Hemmerling TM. Determination of eicosanoid and cytokine production in salvaged blood, stored red blood cell concentrates, and whole blood. J Clin Anesth. 2000; 12:94–99. PMID: 10818321.

68. Gately S, Li WW. Multiple roles of cox-2 in tumor angiogenesis: A target for antiangiogenic therapy. Semin Oncol. 2004; 31:2–11.

69. Mulligan JK, Rosenzweig SA, Young MR. Tumor secretion of vegf induces endothelial cells to suppress t cell functions through the production of pge2. J Immunother. 2010; 33:126–135. PMID: 20145550.

70. Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, et al. Pge (2) contributes to tgf-beta induced t regulatory cell function in human non-small cell lung cancer. Am J Transl Res. 2010; 2:356–367. PMID: 20733946.
71. Soontrapa K, Honda T, Sakata D, Yao C, Hirata T, Hori S, et al. Prostaglandin e2-prostaglandin e receptor subtype 4 (ep4) signaling mediates uv irradiation-induced systemic immunosuppression. Proc Natl Acad Sci U S A. 2011; 108:6668–6673. PMID: 21460251.
72. Bilgin YM, Brand A. Transfusion-related immunomodulation: A second hit in an inflammatory cascade. Vox Sang. 2008; 95:261–271. PMID: 19138255.

73. Seghatchian J. Universal leucodepletion: An overview of some unresolved issues and the highlights of lessons learned. Transfus Apher Sci. 2003; 29:105–117. PMID: 12941346.

74. Baumgartner JM, Nydam TL, Clarke JH, Banerjee A, Silliman CC, McCarter MD. Red blood cell supernatant potentiates lps-induced proinflammatory cytokine response from peripheral blood mononuclear cells. J Interferon Cytokine Res. 2009; 29:333–338. PMID: 19441884.

75. Karam O, Tucci M, Toledano BJ, Robitaille N, Cousineau J, Thibault L, et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion. 2009; 49:2326–2334. PMID: 19624600.

76. Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res. 2006; 135:226–232. PMID: 16926027.

77. Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. Cd4+cd25high regulatory cells in human peripheral blood. J Immunol. 2001; 167:1245–1253. PMID: 11466340.

78. Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory t cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003; 197:403–411. PMID: 12591899.

79. Chen W, Frank ME, Jin W, Wahl SM. Tgf-beta released by apoptotic t cells contributes to an immunosuppressive milieu. Immunity. 2001; 14:715–725. PMID: 11420042.
80. Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000; 405:85–90. PMID: 10811223.

81. Saas P, Bonnefoy F, Kury-Paulin S, Kleinclauss F, Perruche S. Mediators involved in the immunomodulatory effects of apoptotic cells. Transplantation. 2007; 84:S31–S34. PMID: 17632410.

82. Saas P, Angelot F, Bardiaux L, Seilles E, Garnache-Ottou F, Perruche S. Phosphatidylserine-expressing cell byproducts in transfusion: A pro-inflammatory or an anti-inflammatory effect? Transfus Clin Biol. 2012; 19:90–97. PMID: 22677430.

83. McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous tgf-beta in macrophages that have ingested apoptotic cells. J Immunol. 1999; 163:6164–6172. PMID: 10570307.
84. Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978; 299:799–803. PMID: 357971.

85. Williams JG, Hughes LE. Effect of perioperative blood transfusion on recurrence of crohn's disease. Lancet. 1989; 2:131–133. PMID: 2567897.

86. Rizzo JD, Somerfield MR, Hagerty KL, Seidenfeld J, Bohlius J, Bennett CL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 american society of clinical oncology/american society of hematology clinical practice guideline update. J Clin Oncol. 2008; 26:132–149. PMID: 17954713.

87. Cata JP, Chukka V, Wang H, Feng L, Gottumukkala V, Martinez F, et al. Perioperative blood transfusions and survival in patients with non-small cell lung cancer: A retrospective study. BMC Anesthesiol. 2013; 13:42. PMID: 24228905.

88. Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T. Regulation of tumor growth and metastasis: The role of tumor microenvironment. Cancer Growth Metastasis. 2014; 7:9–18. PMID: 24926201.

89. De Oliveira GS Jr., Schink JC, Buoy C, Ahmad S, Fitzgerald PC, McCarthy RJ. The association between allogeneic perioperative blood transfusion on tumour recurrence and survival in patients with advanced ovarian cancer. Transfus Med. 2012; 22:97–103. PMID: 22151920.

90. Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis. Ann Surg. 2012; 256:235–244. PMID: 22791100.
91. Vamvakas EC. Allogeneic blood transfusion and cancer recurrence: 20 years later. Transfusion. 2014; 54:2149–2153. PMID: 25212422.

92. Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013; 24:393–400. PMID: 23433737.

93. Goubran HA, Burnouf T, Stakiw J, Seghatchian J. Platelet microparticle: A sensitive physiological “fine tuning” balancing factor in health and disease. Transfus Apher Sci. 2015; 52:12–18. PMID: 25599988.

94. Goubran H, Sabry W, Kotb R, Seghatchian J, Burnouf T. Platelet microparticles and cancer: An intimate cross-talk. Transfus Apher Sci. 2015; 53:168–172. PMID: 26542350.

95. Drabsch Y, ten Dijke P. Tgf-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012; 31:553–568. PMID: 22714591.
96. Connolly EC, Freimuth J, Akhurst RJ. Complexities of tgf-beta targeted cancer therapy. Int J Biol Sci. 2012; 8:964–978. PMID: 22811618.
97. Morner ME, Edgren G, Martling A, Gunnarsson U, Egenvall M. Preoperative anaemia and perioperative red blood cell transfusion as prognostic factors for recurrence and mortality in colorectal cancer-a swedish cohort study. Int J Colorectal Dis. 2017; 32:223–232. PMID: 27770250.

98. Nathanson SD, Tilley BC, Schultz L, Smith RF. Perioperative allogeneic blood transfusions. Survival in patients with resected carcinomas of the colon and rectum. Arch Surg. 1985; 120:734–738. PMID: 2988481.
99. Weiden PL, Bean MA, Schultz P. Perioperative blood transfusion does not increase the risk of colorectal cancer recurrence. Cancer. 1987; 60:870–874. PMID: 3594405.

100. Donohue JH, Williams S, Cha S, Windschitl HE, Witzig TE, Nelson H, et al. Perioperative blood transfusions do not affect disease recurrence of patients undergoing curative resection of colorectal carcinoma: A mayo/north central cancer treatment group study. J Clin Oncol. 1995; 13:1671–1678. PMID: 7602357.

101. Wiig JN. [blood transfusion in cancer of the colon and rectum. Friend or foe of the surgeon? A review]. Tidsskr Nor Laegeforen. 1990; 110:503–507. PMID: 2408192.
102. Deleplanque G, Deleplanque P, Kraimps JL, Carretier M, Barbier J. [prognostic incidence of blood transfusion in 753 patients operated for colorectal adenocarcinoma]. Ann Chir. 1992; 46:584–591. PMID: 1456687.
103. Faenza A, Cunsolo A, Selleri S, Lucarelli S, Farneti PA, Gozzetti G. Correlation between plasma or blood transfusion and survival after curative surgery for colorectal cancer. Int Surg. 1992; 77:264–269. PMID: 1478807.
104. Chung M, Steinmetz OK, Gordon PH. Perioperative blood transfusion and outcome after resection for colorectal carcinoma. Br J Surg. 1993; 80:427–432. PMID: 8192723.

105. Steup WH, Hojo K, Moriya Y, Sugihara K, Mizuno S, Hermans J, et al. An analysis on the effect of blood transfusion on recurrence and survival in patients undergoing extended lymphadenectomy for colorectal cancer. Hepatogastroenterology. 1994; 41:253–259. PMID: 7959548.
106. Qiu L, Wang DR, Zhang XY, Gao S, Li XX, Sun GP, et al. Impact of perioperative blood transfusion on immune function and prognosis in colorectal cancer patients. Transfus Apher Sci. 2016; 54:235–241. PMID: 26780991.

107. Li XX, Meng J, Sun GP, Tang YX, Liang GF, Wang MF, et al. Effects of perioperative blood transfusion on the prognosis in hereditary and sporadic colon cancer. Biomarkers. 2015; 20:481–486. PMID: 26616149.

108. Kaneko M, Sasaki S, Ishimaru K, Terai E, Nakayama H, Watanabe T. The impact of perioperative allogeneic blood transfusion on survival in elderly patients with colorectal cancer. Anticancer Res. 2015; 35:3553–3558. PMID: 26026124.
109. Tartter PI. Blood transfusion and infectious complications following colorectal cancer surgery. Br J Surg. 1988; 75:789–792. PMID: 3167530.

110. Vamvakas EC, Carven JH, Hibberd PL. Blood transfusion and infection after colorectal cancer surgery. Transfusion. 1996; 36:1000–1008. PMID: 8937412.

111. Schiergens TS, Rentsch M, Kasparek MS, Frenes K, Jauch KW, Thasler WE. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum. 2015; 58:74–82. PMID: 25489697.

112. Hallet J, Tsang M, Cheng ES, Habashi R, Kulyk I, Hanna SS, et al. The impact of perioperative red blood cell transfusions on long-term outcomes after hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2015; 22:4038–4045. PMID: 25752895.

113. Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: A meta-analysis. PLoS One. 2013; 8:e64261. PMID: 23741309.

114. Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999; 23:676–680. PMID: 10390585.

115. Makino Y, Yamanoi A, Kimoto T, El-Assal ON, Kohno H, Nagasue N. The influence of perioperative blood transfusion on intrahepatic recurrence after curative resection of hepatocellular carcinoma. Am J Gastroenterol. 2000; 95:1294–1300. PMID: 10811342.

116. Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: Systematic review and meta-analysis. Int J Surg. 2015; 13:102–110. PMID: 25486261.

117. Fong Y, Karpeh M, Mayer K, Brennan MF. Association of perioperative transfusions with poor outcome in resection of gastric adenocarcinoma. Am J Surg. 1994; 167:256–260. PMID: 8135315.

118. Hyung WJ, Noh SH, Shin DW, Huh J, Huh BJ, Choi SH, et al. Adverse effects of perioperative transfusion on patients with stage iii and iv gastric cancer. Ann Surg Oncol. 2002; 9:5–12. PMID: 11829431.

119. Kosumi K, Baba Y, Harada K, Yoshida N, Watanabe M, Baba H. Perioperative blood transfusion, age at surgery, and prognosis in a database of 526 upper gastrointestinal cancers. Dig Surg. 2015; 32:445–453. PMID: 26550999.

120. Dresner SM, Lamb PJ, Shenfine J, Hayes N, Griffin SM. Prognostic significance of peri-operative blood transfusion following radical resection for oesophageal carcinoma. Eur J Surg Oncol. 2000; 26:492–497. PMID: 11016472.

121. Langley SM, Alexiou C, Bailey DH, Weeden DF. The influence of perioperative blood transfusion on survival after esophageal resection for carcinoma. Ann Thorac Surg. 2002; 73:1704–1709. PMID: 12078756.

122. Reeh M, Ghadban T, Dedow J, Vettorazzi E, Uzunoglu FG, Nentwich M, et al. Allogenic blood transfusion is associated with poor perioperative and long-term outcome in esophageal cancer. World J Surg. 2017; 41:208–215. PMID: 27730355.

123. Lim MC, Kim JY, Kim TH, Park S, Kong SY, Yoon JH, et al. Allogeneic blood transfusion given before radiotherapy is associated with the poor clinical outcome in patients with cervical cancer. Yonsei Med J. 2008; 49:993–1003. PMID: 19108024.

124. Santin AD, Bellone S, Parrish RS, Coke C, Dunn D, Roman J, et al. Influence of allogeneic blood transfusion on clinical outcome during radiotherapy for cancer of the uterine cervix. Gynecol Obstet Invest. 2003; 56:28–34. PMID: 12867765.

125. Linder BJ, Frank I, Cheville JC, Tollefson MK, Thompson RH, Tarrell RF, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol. 2013; 63:839–845. PMID: 23332883.

126. Little AG, Wu HS, Ferguson MK, Ho CH, Bowers VD, Segalin A, et al. Perioperative blood transfusion adversely affects prognosis of patients with stage i non-small-cell lung cancer. Am J Surg. 1990; 160:630–632. discussion 3. PMID: 2174651.

127. Perisanidis C, Dettke M, Papadogeorgakis N, Schoppmann A, Mittlbock M, Kyzas PA, et al. Transfusion of allogenic leukocyte-depleted packed red blood cells is associated with postoperative morbidity in patients undergoing oral and oropharyngeal cancer surgery. Oral Oncol. 2012; 48:372–378. PMID: 22182932.

128. Karakida K, Aoki T, Ota Y, Yamazaki H, Otsuru M, Takahashi M, et al. Analysis of risk factors for surgical-site infections in 276 oral cancer surgeries with microvascular free-flap reconstructions at a single university hospital. J Infect Chemother. 2010; 16:334–339. PMID: 20809241.

129. Liu SA, Wong YK, Poon CK, Wang CC, Wang CP, Tung KC. Risk factors for wound infection after surgery in primary oral cavity cancer patients. Laryngoscope. 2007; 117:166–171. PMID: 17202947.

130. Taniguchi Y, Okura M. Prognostic significance of perioperative blood transfusion in oral cavity squamous cell carcinoma. Head Neck. 2003; 25:931–936. PMID: 14603453.

131. Szakmany T, Dodd M, Dempsey GA, Lowe D, Brown JS, Vaughan ED, et al. The influence of allogenic blood transfusion in patients having free-flap primary surgery for oral and oropharyngeal squamous cell carcinoma. Br J Cancer. 2006; 94:647–653. PMID: 16523195.

132. Chau JK, Harris JR, Seikaly HR. Transfusion as a predictor of recurrence and survival in head and neck cancer surgery patients. J Otolaryngol Head Neck Surg. 2010; 39:516–522. PMID: 20828514.
133. Ervens J, Marks C, Hechler M, Plath T, Hansen D, Hoffmeister B. Effect of induced hypotensive anaesthesia vs isovolaemic haemodilution on blood loss and transfusion requirements in orthognathic surgery: A prospective, single-blinded, randomized, controlled clinical study. Int J Oral Maxillofac Surg. 2010; 39:1168–1174. PMID: 20961738.

134. Choi WS, Samman N. Risks and benefits of deliberate hypotension in anaesthesia: A systematic review. Int J Oral Maxillofac Surg. 2008; 37:687–703. PMID: 18511238.

135. Varol A, Basa S, Ozturk S. The role of controlled hypotension upon transfusion requirement during maxillary downfracture in double-jaw surgery. J Craniomaxillofac Surg. 2010; 38:345–349. PMID: 19913434.

136. Zellin G, Rasmusson L, Palsson J, Kahnberg KE. Evaluation of hemorrhage depressors on blood loss during orthognathic surgery: A retrospective study. J Oral Maxillofac Surg. 2004; 62:662–666. PMID: 15170275.

137. Sankar D, Krishnan R, Veerabahu M, Vikraman B. Evaluation of the efficacy of tranexamic acid on blood loss in orthognathic surgery. A prospective, randomized clinical study. Int J Oral Maxillofac Surg. 2012; 41:713–717. PMID: 22340993.

138. Ramos E, Dalmau A, Sabate A, Lama C, Llado L, Figueras J, et al. Intraoperative red blood cell transfusion in liver transplantation: Influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003; 9:1320–1327. PMID: 14625833.

139. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002; 74:1180–1186. PMID: 12400765.

140. Kleczynski P, Dziewierz A, Bagienski M, Rzeszutko L, Sorysz D, Trebacz J, et al. Association between blood transfusions and 12-month mortality after transcatheter aortic valve implantation. Int Heart J. 2017; 58:50–55. PMID: 28077819.

141. Bursi F, Barbieri A, Politi L, Di Girolamo A, Malagoli A, Grimaldi T, et al. Perioperative red blood cell transfusion and outcome in stable patients after elective major vascular surgery. Eur J Vasc Endovasc Surg. 2009; 37:311–318. PMID: 19111480.

142. Weber EW, Slappendel R, Prins MH, van der Schaaf DB, Durieux ME, Strumper D. Perioperative blood transfusions and delayed wound healing after hip replacement surgery: Effects on duration of hospitalization. Anesth Analg. 2005; 100:1416–1421. table of contents. PMID: 15845698.

143. Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 u to 2 u packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009; 208:931–937. 7.e1–7.e2. discussion 8-9. PMID: 19476865.

144. Ferraris VA, Davenport DL, Saha SP, Austin PC, Zwischenberger JB. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012; 147:49–55. PMID: 22250113.

145. de Almeida JP, Vincent JL, Galas FR, de Almeida EP, Fukushima JT, Osawa EA, et al. Transfusion requirements in surgical oncology patients: A prospective, randomized controlled trial. Anesthesiology. 2015; 122:29–38. PMID: 25401417.
146. Samman N, Cheung LK, Tong AC, Tideman H. Blood loss and transfusion requirements in orthognatic surgery. J Oral Maxillofac Surg. 1996; 54:21–24. discussion 5-6. PMID: 8530995.
147. Gong SG, Krishnan V, Waack D. Blood transfusions in bimaxillary orthognathic surgery: Are they necessary? Int J Adult Orthodon Orthognath Surg. 2002; 17:314–317. PMID: 12593003.
148. Ueki K, Marukawa K, Shimada M, Nakagawa K, Yamamoto E. The assessment of blood loss in orthognathic surgery for prognathia. J Oral Maxillofac Surg. 2005; 63:350–354. PMID: 15742286.

149. Stewart A, Newman L, Sneddon K, Harris M. Aprotinin reduces blood loss and the need for transfusion in orthognathic surgery. Br J Oral Maxillofac Surg. 2001; 39:365–370. PMID: 11601817.
150. Moenning JE, Bussard DA, Lapp TH, Garrison BT. Average blood loss and the risk of requiring perioperative blood transfusion in 506 orthognathic surgical procedures. J Oral Maxillofac Surg. 1995; 53:880–883. PMID: 7629615.

151. Kramer FJ, Baethge C, Swennen G, Teltzrow T, Schulze A, Berten J, et al. Intra- and perioperative complications of the lefort i osteotomy: A prospective evaluation of 1000 patients. J Craniofac Surg. 2004; 15:971–977. discussion 8-9. PMID: 15547385.

152. Flood TR, Ilankovan V, Moos KF, el-Attar A. Cross-match requirements in orthognathic surgery: An audit. Br J Oral Maxillofac Surg. 1990; 28:292–294. PMID: 2248935.

153. Tang ZL, Wang X, Yi B, Li ZL, Liang C, Wang XX. Effects of the preoperative administration of yunnan baiyao capsules on intraoperative blood loss in bimaxillary orthognathic surgery: A prospective, randomized, double-blind, placebo-controlled study. Int J Oral Maxillofac Surg. 2009; 38:261–266. PMID: 19153029.

154. Faverani LP, Ramalho-Ferreira G, Fabris AL, Polo TO, Poli GH, Pastori CM, et al. Intraoperative blood loss and blood transfusion requirements in patients undergoing orthognathic surgery. Oral Maxillofac Surg. 2014; 18:305–310. PMID: 23620250.

155. Garcia-Roa M, Del Carmen Vicente-Ayuso M, Bobes AM, Pedraza AC, Gonzalez-Fernandez A, Martin MP, et al. Red blood cell storage time and transfusion: Current practice, concerns and future perspectives. Blood Transfus. 2017; 15:222–231. PMID: 28518049.
156. Cancelas JA, Dumont LJ, Maes LA, Rugg N, Herschel L, Whitley PH, et al. Additive solution-7 reduces the red blood cell cold storage lesion. Transfusion. 2015; 55:491–498. PMID: 25233911.

157. Hess JR, Rugg N, Knapp AD, Gormas JF, Silberstein EB, Greenwalt TJ. Successful storage of rbcs for 9 weeks in a new additive solution. Transfusion. 2000; 40:1007–1011. PMID: 10960530.

158. Antonelou MH, Kriebardis AG, Papassideri IS. Aging and death signalling in mature red cells: From basic science to transfusion practice. Blood Transfus. 2010; 8(Suppl 3):s39–s47. PMID: 20606748.
159. Blasi B, D'Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012; 22:90–96. PMID: 22394111.

160. D'Alessandro A, D'Amici GM, Vaglio S, Zolla L. Time-course investigation of sagm-stored leukocyte-filtered red bood cell concentrates: From metabolism to proteomics. Haematologica. 2012; 97:107–115. PMID: 21993682.
161. Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: Structural aspects and implications for transfusion. Transfus Med. 2008; 18:335–347. PMID: 19140816.
162. Hod EA, Godbey EA. The outsider adverse event in transfusion: Inflammation. Presse Med. 2016; 45:e325–e329. PMID: 27476779.

164. Eikelboom JW, Cook RJ, Barty R, Liu Y, Arnold DM, Crowther MA, et al. Rationale and design of the informing fresh versus old red cell management (inform) trial: An international pragmatic randomized trial. Transfus Med Rev. 2016; 30:25–29. PMID: 26651419.

165. Shah A, McKechnie S, Brunskill SJ, Stanworth SJ. Fresh versus old red cell transfusions: What have the recent clinical trials found. Curr Opin Hematol. 2016; 23:550–556. PMID: 27518928.
166. Chalfin HJ, Frank SM, Feng Z, Trock BJ, Drake CG, Partin AW, et al. Allogeneic versus autologous blood transfusion and survival after radical prostatectomy. Transfusion. 2014; 54:2168–2174. PMID: 24601996.
167. Dietrich W, Thuermel K, Heyde S, Busley R, Berger K. Autologous blood donation in cardiac surgery: Reduction of allogeneic blood transfusion and cost-effectiveness. J Cardiothorac Vasc Anesth. 2005; 19:589–596. PMID: 16202891.

168. Heiss MM, Mempel W, Delanoff C, Jauch KW, Gabka C, Mempel M, et al. Blood transfusion-modulated tumor recurrence: First results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994; 12:1859–1867. PMID: 8083709.

169. Oh AY, Seo KS, Lee GE, Kim HJ. Effect of preoperative autologous blood donation on patients undergoing bimaxillary orthognathic surgery: A retrospective analysis. Int J Oral Maxillofac Surg. 2016; 45:486–489. PMID: 26678802.

170. Jakovina Blazekovic S, Bicanic G, Hrabac P, Tripkovic B, Delimar D. Pre-operative autologous blood donation versus no blood donation in total knee arthroplasty: A prospective randomised trial. Int Orthop. 2014; 38:341–346. PMID: 24305788.

171. Zhou J. A review of the application of autologous blood transfusion. Braz J Med Biol Res. 2016; 49:e5493. PMID: 27533770.

172. Practice guidelines for blood component therapy: A report by the american society of anesthesiologists task force on blood component therapy. Anesthesiology. 1996; 84:732–747. PMID: 8659805.
173. Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The international study of perioperative transfusion. Anesth Analg. 1998; 86:9–15. PMID: 9428843.
174. Barile L, Fominskiy E, Di Tomasso N, Alpizar Castro LE, Landoni G, De Luca M, et al. Acute normovolemic hemodilution reduces allogeneic red blood cell transfusion in cardiac surgery: A systematic review and meta-analysis of randomized trials. Anesth Analg. 2017; 124:743–752. PMID: 27669554.
175. Lee JM, Seo KS, Kim HJ, Shin SY. Experience of a bloodless two-jaw surgery and care in jehovah's witnesses with anemia. J Korean Dent Soc Anesthesiol. 2012; 12:25–31.

176. Ickx BE, Rigolet M, Van Der Linden PJ. Cardiovascular and metabolic response to acute normovolemic anemia. Effects of anesthesia. Anesthesiology. 2000; 93:1011–1016. PMID: 11020756.
177. Hill J, James V. Survey of autologous blood transfusion activity in england (2001). Transfus Med. 2003; 13:9–15. PMID: 12581449.

178. Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010; 105:401–416. PMID: 20802228.

Table 1
Recent recommendations and clinical guidelines on the threshold for a red blood cell transfusion
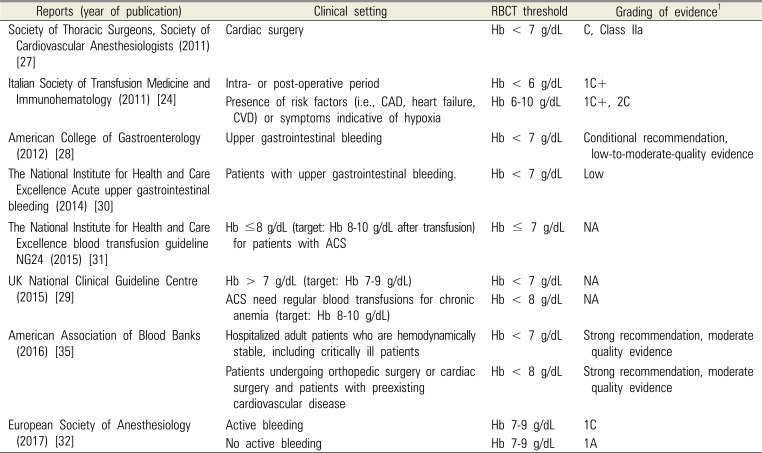
| Reports (year of publication) | Clinical setting | RBCT threshold | Grading of evidence1 |
|---|---|---|---|
| Society of Thoracic Surgeons, Society of Cardiovascular Anesthesiologists (2011) [27] | Cardiac surgery | Hb < 7 g/dL | C, Class IIa |
| Italian Society of Transfusion Medicine and Immunohematology (2011) [24] | Intra- or post-operative period | Hb < 6 g/dL | 1C+ |
| Presence of risk factors (i.e., CAD, heart failure, CVD) or symptoms indicative of hypoxia | Hb 6-10 g/dL | 1C+, 2C | |
| American College of Gastroenterology (2012) [28] | Upper gastrointestinal bleeding | Hb < 7 g/dL | Conditional recommendation, low-to-moderate-quality evidence |
| The National Institute for Health and Care Excellence Acute upper gastrointestinal bleeding (2014) [30] | Patients with upper gastrointestinal bleeding. | Hb < 7 g/dL | Low |
| The National Institute for Health and Care Excellence blood transfusion guideline NG24 (2015) [31] | Hb ≤8 g/dL (target: Hb 8-10 g/dL after transfusion) for patients with ACS | Hb ≤ 7 g/dL | NA |
| UK National Clinical Guideline Centre (2015) [29] | Hb > 7 g/dL (target: Hb 7-9 g/dL) | Hb < 7 g/dL | NA |
| ACS need regular blood transfusions for chronic anemia (target: Hb 8-10 g/dL) | Hb < 8 g/dL | NA | |
| American Association of Blood Banks (2016) [35] | Hospitalized adult patients who are hemodynamically stable, including critically ill patients | Hb < 7 g/dL | Strong recommendation, moderate quality evidence |
| Patients undergoing orthopedic surgery or cardiac surgery and patients with preexisting cardiovascular disease | Hb < 8 g/dL | Strong recommendation, moderate quality evidence | |
| European Society of Anesthesiology (2017) [32] | Active bleeding | Hb 7-9 g/dL | 1C |
| No active bleeding | Hb 7-9 g/dL | 1A |
Table 2
Blood loss and perioperative data in orthognathic surgery
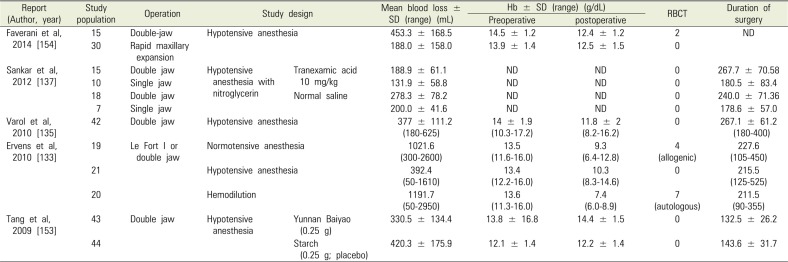
| Report (Author, year) | Study population | Operation | Study design | Mean blood loss ± SD (range) (mL) | Hb ± SD (range) (g/dL) | RBCT | Duration of surgery | ||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | postoperative | ||||||||
| Faverani et al, 2014 [154] | 15 | Double-jaw | Hypotensive anesthesia | 453.3 ± 168.5 | 14.5 ± 1.2 | 12.4 ± 1.2 | 2 | ND | |
| 30 | Rapid maxillary expansion | 188.0 ± 158.0 | 13.9 ± 1.4 | 12.5 ± 1.5 | 0 | ||||
| Sankar et al, 2012 [137] | 15 | Double jaw | Hypotensive anesthesia with nitroglycerin | Tranexamic acid 10 mg/kg | 188.9 ± 61.1 | ND | ND | 0 | 267.7 ± 70.58 |
| 10 | Single jaw | 131.9 ± 58.8 | ND | ND | 0 | 180.5 ± 83.4 | |||
| 18 | Double jaw | Nornal saline | 278.3 ± 78.2 | ND | ND | 0 | 240.0 ± 71.36 | ||
| 7 | Single jaw | 200.0 ± 41.6 | ND | ND | 0 | 178.6 ± 57.0 | |||
| Varol et al, 2010 [135] | 42 | Double jaw | Hypotensive anesthesia | 377 ± 111.2 (180-625) | 14 ± 1.9 (10.3-17.2) | 11.8 ± 2 (8.2-16.2) | 0 | 267.1 ± 61.2 (180-400) | |
| Ervens et al, 2010 [133] | 19 | Le Fort I or double jaw | Normotensive anesthesia | 1021.6 (300-2600) | 13.5 (11.6-16.0) | 9.3 (6.4-12.8) | 4 (allogenic) | 227.6 (105-450) | |
| 21 | Hypotensive anesthesia | 392.4 (50-1610) | 13.4 (12.2-16.0) | 10.3 (8.3-14.6) | 0 | 215.5 (125-525) | |||
| 20 | Hemodilution | 1191.7 (50-2950) | 13.6 (11.3-16.0) | 7.4 (6.0-8.9) | 7 (autologous) | 211.5 (90-355) | |||
| Tang et al, 2009 [153] | 43 | Double jaw | Hypotensive anesthesia | Yunnan Baiyao (0.25 g) | 330.5 ± 134.4 | 13.8 ± 16.8 | 14.4 ± 1.5 | 0 | 132.5 ± 26.2 |
| 44 | Starch (0.25 g; placebo) | 420.3 ± 175.9 | 12.1 ±1.4 | 12.2 ± 1.4 | 0 | 143.6 ± 31.7 | |||




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download