Abstract
Issues related to the control of seizures and bleeding, as well as behavioral management due to mental retardation, render dental treatment less accessible or impossible for patients with Sturge-Weber syndrome (SWS). A 41-year-old man with SWS visited a dental clinic for rehabilitation of missing dentition. A bilateral port-wine facial nevus and intraoral hemangiomatous swollen lesion of the left maxillary and mandibular gingivae, mucosa, and lips were noted. The patient exhibited extreme anxiety immediately after injection of a local anesthetic and required various dental treatments to be performed over multiple visits. Therefore, full-mouth rehabilitation over two visits with general anesthesia and two visits with target-controlled intravenous infusion of a sedative anesthesia were planned. Despite concerns regarding seizure control, bleeding control, and airway management, no specific complications occurred during the treatments, and the patient was satisfied with the results.
Sturge-Weber syndrome (SWS) is characterized by a facial port-wine nevus and ipsilateral leptomeningeal angiomatosis and is one of the most common neurocutaneous syndromes. Patients with SWS typically present with neurologic features of epileptic seizures and intellectual impairment, but less often may also exhibit hemiparesis, hemiatrophy, and visual-field defects (i.e., glaucoma) [1]. An ipsilateral facial cutaneous vascular malformation usually affects the upper face in a distribution consistent with the ophthalmic division of the trigeminal nerve, probably as a result of the abnormal persistence of an embryologic venous plexus [23]. Intraoral angiomatosis can involve the lips, causing macrocheilia and resulting in hemihypertrophy of the buccal mucosa, palate, and floor of the mouth [4]. Gingival enlargement can result either from angiomatous proliferation or as a reaction to the anticonvulsants used by patients with epileptic seizures, and it may be further compounded by poor oral hygiene secondary to mental retardation [567].
To date, oral management has focused on the control of bleeding associated with a hemangioma. However, the dental problems suffered by SWS patients include oral angiomatosis as well as malocclusion and poor oral hygiene, and they are often in need of comprehensive and complex dental treatment. The necessity for seizure control, bleeding control, and behavior management due to these patients' mental disabilities renders dental treatment at a local clinic less accessible, and sometimes even impossible in these patients. Specialized dental care combined with various types of sedation and general anesthesia are useful for SWS patients, although there are some unique considerations and associated risks.
Fear and stress can increase the blood pressure in the brain and eye, and in SWS patients, it is particularly important that these elevations are controlled. Anesthetic management can reduce the levels of fear and stress, and in SWS patients, the appropriate approach is determined by observing the clinical manifestations of localized superficial skin lesions and the extent of systemic involvement, evaluating the associated anomalies, anticipating problems with intubation due to the presence of angiomas of the mouth and upper airway, and recording concurrent therapies [8].
In this report, we describe a case of SWS in which comprehensive oral rehabilitation was achieved using a mixture of both general anesthesia and sedation.
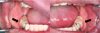
A 41-year-old man with SWS visited the dental hospital requesting rehabilitation of missing dentition. Brain CT revealed cortical atrophy and dystrophic gyral calcification (cortical venous angioma) involving the right frontoparietal lobe. An extraoral examination revealed that the typical feature of port-wine facial nevus was distributed bilaterally from his forehead to his chin and was present intraorally, involving the left maxillary and mandibular gingivae, mucosa, and lips. A marked left-side nevus covered the patient's entire face from his forehead to his chin, and a right-side nevus covered part of his forehead and his entire maxilla and circumoral area (Fig. 1A). Other conspicuous features included swollen cheeks, and upper- and lower-lip enlargement, particularly on the left side. The patient had undergone glaucoma surgery, but he had lost the sight in his left eye. Additionally, he experienced discomfort when moving his left arm and fingers. He was able to communicate with other people, but he exhibited some mental retardation, as well as extreme fear and anxiety about undergoing dental treatment. He was taking an anticonvulsant to control his epilepsy, but he was not taking any anticoagulant.
Intraoral examination revealed generalized gingival swelling and redness, and most of his remaining teeth were salvageable; exceptions were the maxillary right canine, first and second premolars, left second premolar, mandibular left canine, second premolar, right canine, and first premolar. Gingival overgrowth was indistinct, but the half ridges on the left side were violet-red in color, had nonkeratinized gingiva, and blanched on pressure application, suggesting an angiomatous lesion. His lower buccal vestibules were missing, and there was no attached gingiva, indicating that there was insufficient denture support (Fig. 1B-F, Fig. 2).
Given that the patient had good treatment compliance, sectional tooth extraction was attempted. However, he displayed extreme anxiety immediately after the injection of a local anesthetic, and the procedure was suspended out of concern for his blood pressure. The patient was in need of multiple extractions, endodontic treatment, and prosthodontic treatment. Given the circumstances, full-mouth rehabilitation with a minimal number of dental visits using general and sedative anesthesia was planned, including two visits with general anesthesia and two with sedation. Before surgery, the patient underwent blood tests, chest radiography, and risk assessment. The patient and his guardian provided written informed consent to the planned procedures.
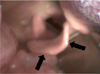
The patient was instructed to maintain his routine medication. His EKG, heart rate, blood pressure, respiratory rate, end-tidal carbon dioxide (CO2), body temperature, and entropy level were all monitored throughout the procedures. An intravenous (IV) line was secured under 4 L/min oxygen (O2) and nitrous oxide (N2O) gas (administered via a face mask); 4% sevoflurane gas and atracurium (IV 25 mg) were also administered. The physician anticipated the possibilities of laryngeal edema and a problematic intubation associated with this syndrome. Under an adequate depth of anesthesia, the epiglottis was checked with a bronchoscope and oral camera, and gentle nasal intubation using a Mac #3 laryngoscope was performed (Fig. 3). The trachea was successfully intubated with a 26-mm endotracheal tube (ETT), with no contact bleeding. Anesthesia was maintained with 2 L/min O2, N2O, and 2% sevoflurane. During the operation, atropine (0.5 mg) was injected in response to a lowered heart rate; no other specific events occurred (Table 1).
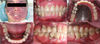
At the first visit, scaling, extraction of 12 teeth, and endodontic treatment of the maxillary left second premolar and mandibular left canine were performed under general anesthesia. Crown preparation, final impressions, and provisionalization of eight teeth for fixed dental prostheses (FDPs) were achieved at the second visit, also under general anesthesia. Because it was not necessary to spray water into the mouth and the required operation time was short, FDP and temporary denture delivery were achieved at the third visit under target-controlled infusion IV sedation using Orchestra (Fresinius Vial, France). At the fourth visit, 2 months later, final impressions for removable partial dentures were made under IV sedation. Three more visits were needed for denture delivery (Figs. 4, 5); by that time, the patient had become accustomed to the circumstances and medical team and was readily sedated such that there was almost no need for propofol. At the first session with sedation, the anticonvulsant premedication was skipped due to the patient's misunderstanding. The patient had complained of experiencing an epileptic aura during the anesthetic induction state; midazolam (IV 5 mg) was immediately administered, and the symptom subsided. Throughout the procedure, the patient exhibited no specific complications and was discharged 1 hour after stabilization.
Denture fabrication was rendered difficult in this patient due to the presence of insufficient supporting tissue, vestiges of hemangioma, and enlarged soft tissue (Fig. 6). Provisional dentures were applied for approximately 2 months, and the soft-tissue replacement and border margins were checked. The patient had hemiparesis on the left side, and he was educated over multiple sessions about how to make adjustments when wearing and removing his dentures. Direct retainers were made using wrought wire for further adjustment. Follow-ups were conducted 2 days, 1 week, 1 month, 7 months, and 14 months after denture delivery. Border adjustments were made, and slight loosening of the retainer loosening was observed. No other specific complications were observed, and the patient was satisfied with the results.
Oral manifestations of SWS can vary considerably, and changes in the morphology and histology of the gingiva, periodontium, and even the pulp have been reported. The most common feature is gingival hemangiomatous lesion, which is usually restricted to the ipsilateral maxilla, mandible, floor of the mouth, lips, cheeks, palate, and tongue [5]. Most reports on dental treatment in these patients have focused on periodontal management associated with bleeding control. When oral surgical procedures such as periodontal surgery or tooth extraction are needed in the area affected by vascular lesions, special attention must be paid to the risk of severe intra- and postoperative hemorrhage [9]. In the case of mild-to-moderate gingival inflammation or enlargement, suggested conservative measures include oral hygiene orientation, oral prophylaxis, use of a 0.12% chlorhexidine mouthwash, and patient motivation. All of these procedures significantly reduce inflammation and tissue growth without the necessity of an invasive surgical intervention [6]. The case reported herein presented with mild swelling involving the upper maxillary gingiva that blanched upon the application of pressure. Therefore, proper patient management and reduction of gingival inflammation using an antimicrobial dressing were used to avoid massive bleeding. The extraction sockets were packed with a collagen sponge (CollaDerm, Bioland, Chungbuk, Korea) and sutured. In this case, the most critical requirement was to control the patient's panic. Excessive anxiety or stress will result in increased blood pressure, which in turn will induce enlargement of the hemangioma or increase the intraocular pressure. Specific approaches to stress management during dental treatment in patients with SWS have not been reported. Sedative treatments that are administered to dentophobics or mentally retarded patients could also be applied to SWS patients, not only with an aim of reducing their levels of anxiety, but also to minimize the required number of dental visits.
Anesthesia should be planned to avoid trauma to the hemangioma and elevations in intraocular and intracranial pressure in SWS patients [10]. Furthermore, these patients should be carefully evaluated for associated anomalies. Mask ventilation, laryngoscopy, and intubation may be difficult due to angiomas involving the nose, lips, oral cavity, tongue, palate, larynx, and trachea [101112]. Intubation may be difficult due to angiomas of the mouth and upper airway, which have been reported to cause hypertrophy of the involved areas as well as the nasopharynx. Such problems with intubation in SWS patients have been reported previously [12]. Routine application of an algorithmic approach to failed intubation is associated with much higher salvage success rates than those achieved historically [13]. Several major guidelines are now available for the management of difficulties associated with the airway [1415]; laryngeal mask airway or fiber-optic tracheal intubation should be performed. Furthermore, because uncontrolled hemorrhage may result from perforation or rupture of these vascular lesions, intubation should be conducted using a soft, well-lubricated, nonstyleted, cuffed ETT, and careful oropharyngeal and tracheobronchial suction is crucial to avoiding trauma to these lesions. A smooth induction, intubation, and extubation of these patients is also mandatory to limit inadvisable elevations in the intraocular and intracranial pressure. A light level of anesthesia, bucking, straining, and airway obstruction during these periods are detrimental to the patient. Drugs such as suxamethonium and ketamine, which are known to induce elevations in intraocular pressure, should also be avoided. While dental procedures can be applied to SWS patients, some important considerations are needed in such procedures due to the characteristic oral conditions of these patients. Their enlarged buccal cheeks and tongue can make it difficult to take denture impressions and to seat the dentures; the loss of attached gingiva causes insufficient denture support, and the intraoral hemangiomas can cause massive bleeding as a result of minor injuries. Amblyopia and hemiparesis in some patients make it difficult for them to handle dentures. The use of temporary dentures for a prolonged time may enable the physician to train the patient in denture management, determine the denture supporting/relief area, and enable adaptation of the enlarged tissues. Mucosal changes could occur along the hemangioma following completion of treatment, and so regular checkups are essential to enable adjustment of the denture base to prevent soreness.
Figures and Tables
Fig. 1
(A) Extraoral photograph showing a marked facial nevus, (B-F) Intraoral photographs obtained during the patient's first visit. The arrows indicate the hemangioma in the gingiva.
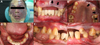
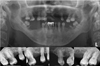
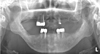
Fig. 2
Panoramic radiograph and intraoral radiographs obtained during the patient's first visit revealing missing dentition and advanced dental caries.
References
2. Taly AB, Nagaraja D, Das S, Shankar SK, Pratibha NG. Sturge-weber-dimitri disease without facial nevus. Neurology. 1987; 37:1063–1064.

3. Thomas-Sohl KA, Vaslow DF, Maria BL. Sturge-weber syndrome: A review. Pediatr Neurol. 2004; 30:303–310.

4. Suprabha BS, Baliga M. Total oral rehabilitation in a patient with portwine stains. J Indian Soc Pedod Prev Dent. 2005; 23:99–102.

5. Caiazzo A, Mehra P, Papageorge MB. The use of preoperative percutaneous transcatheter vascular occlusive therapy in the management of sturge-weber syndrome: Report of a case. J Oral Maxillofac Surg. 1998; 56:775–778.

6. Pontes FS, Conte N, da Costa RM, Loureiro AM, do Nascimento LS, Pontes HA. Periodontal growth in areas of vascular malformation in patients with sturge-weber syndrome: A management protocol. J Craniofac Surg. 2014; 25:e1–e3.
7. Yamashiro M, Furuya H. Anesthetic management of a patient with sturge-weber syndrome undergoing oral surgery. Anesth Prog. 2006; 53:17–19.

8. Delvi MB, Takrouri MS. Anesthesia for encephalo-trigeminal angiomatosis (sturge-weber syndrome). Middle East J Anaesthesiol. 2006; 18:785–790.
9. Perez DE, Pereira Neto JS, Graner E, Lopes MA. Sturge-weber syndrome in a 6-year-old girl. Int J Paediatr Dent. 2005; 15:131–135.

10. Batra RK, Gulaya V, Madan R, Trikha A. Anaesthesia and the sturge-weber syndrome. Can J Anaesth. 1994; 41:133–136.

11. Butler MG, Hayes BG, Hathaway MM, Begleiter ML. Specific genetic diseases at risk for sedation/anesthesia complications. Anesth Analg. 2000; 91:837–855.

12. Wong HS, Abdul Rahman R, Choo SY, Yahya N. Sturge-weber-syndrome with extreme ocular manifestation and rare association of upper airway angioma with anticipated difficult airway. Med J Malaysia. 2012; 67:435–437.
13. Crosby ET. An evidence-based approach to airway management: Is there a role for clinical practice guidelines? Anaesthesia. 2011; 66:Suppl 2. 112–118.

14. American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: An updated report by the american society of anesthesiologists task force on management of the difficult airway. Anesthesiology. 2003; 98:1269–1277.
15. Henderson JJ, Popat MT, Latto IP, Pearce AC. Difficult Airway Society. Difficult airway society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004; 59:675–694.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download