Abstract
Background
Methods
Results
Conclusion
Acknowledgments
References
Fig. 1
A heatmap illustrating detailed information on adopting modern technologies (DTI, neuro-navigation and IOM), the extent of tumor resection and postoperative neurological status. DTI, diffusion tensor imaging; IOM, intraoperative neurophysiological monitoring; GTR, gross total resection; STR, subtotal resection; PA, partial resection.
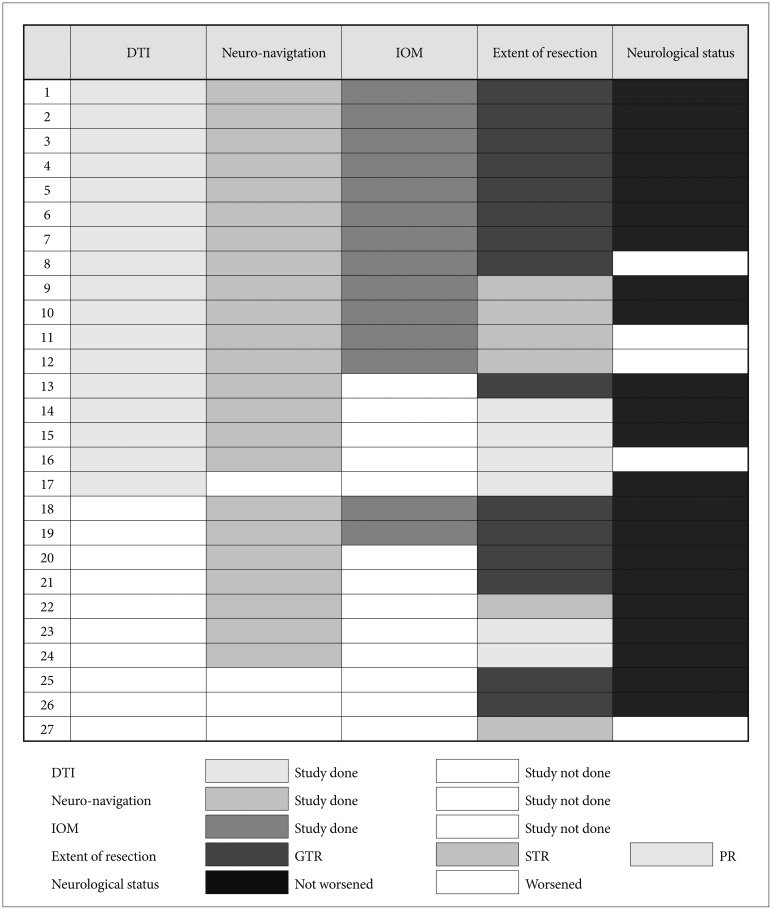
Fig. 2
Kaplan-Meier plots for PFS and OS of 37 patients according to histological diagnosis (A and D), the extent of tumor resection (B and E), and adjuvant therapy (C and F). PFS, progression-free survival; OS, overall survival; GTR, gross total resection; STR, subtotal resection; PA, partial resection; GBL, glioblastoma, IDH-wildtype; AA, anaplastic astrocytoma, IDH-wildtype; DMG, diffuse midline glioma, H3 K27M-mutant; CNS, central nervous system; PA, pilocytic astrocytoma.
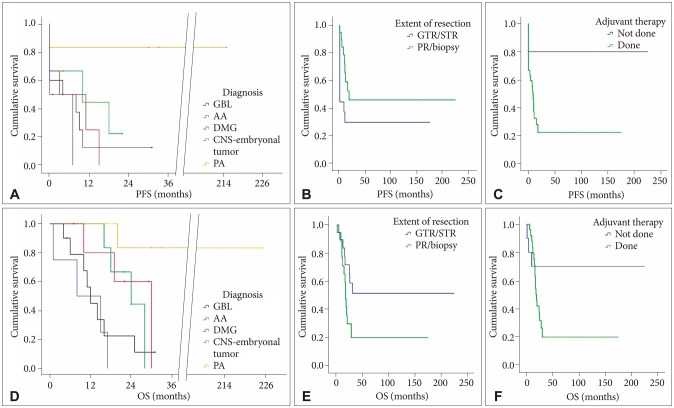
Fig. 3
Demonstration of planning of surgical approach via preoperative DTI. Coronal MRI (A) and DTI (B) show left thalamic tumor with anteromedially displaced thalamus and laterally deviated PLIC. The transparietal approach was adopted, and gross total resection was performed (C). Coronal MRI (D) and DTI (E) show a right thalamic tumor with anteromedially displaced thalamus and anteromedially deviated PLIC. Tumor was radically removed via a transtemporal approach (F). DTI, diffusion tensor imaging; PLIC, posterior limb of internal capsule.
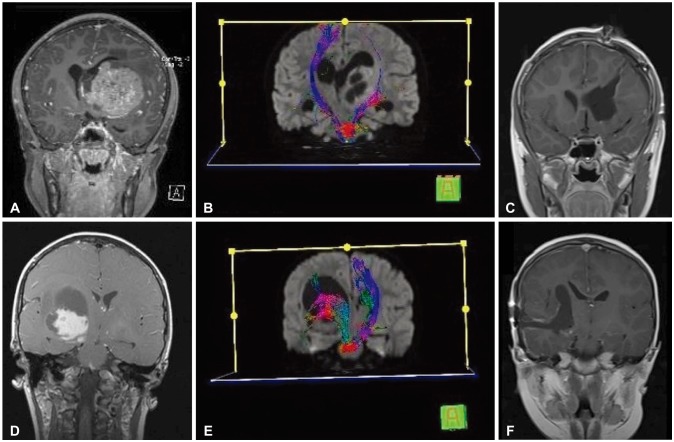
Table 1
Location and extent of the tumors
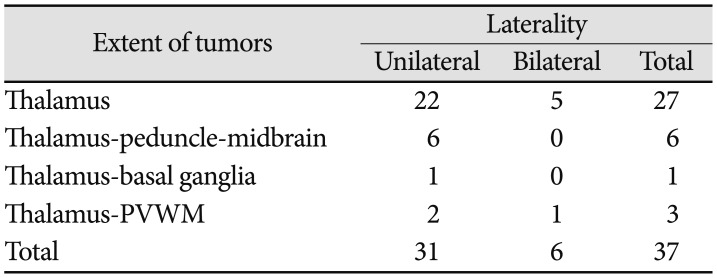
| Extent of tumors | Laterality | ||
|---|---|---|---|
| Unilateral | Bilateral | Total | |
| Thalamus | 22 | 5 | 27 |
| Thalamus-peduncle-midbrain | 6 | 0 | 6 |
| Thalamus-basal ganglia | 1 | 0 | 1 |
| Thalamus-PVWM | 2 | 1 | 3 |
| Total | 31 | 6 | 37 |
Table 2
DTI findings and surgical approaches planned by the DTI finding
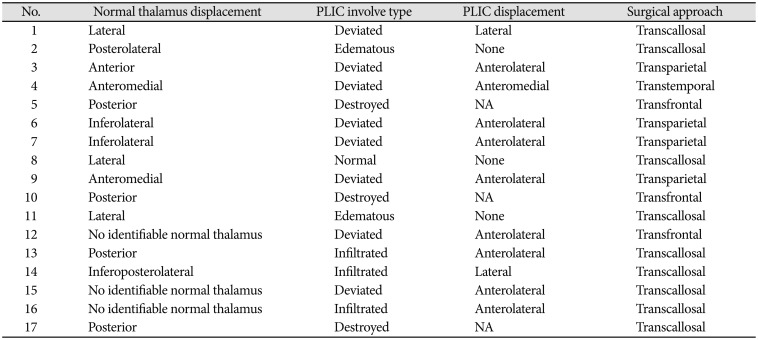
Staged tumor resections were performed in one patient (Case 6, 7) (Case 10, 17) (Case 12, 15). Deviated: normal or only slightly decreased fractional anisotropy (FA) with abnormal location and/or direction resulting from bulk mass displacement. Edematous: substantially decreased FA with normal location and direction (i.e., normal hues on directional color maps). Infiltrated: substantially decreased FA with abnormal hues on directional color maps. Destroyed: isotropic (or near isotropic) diffusion such that the tract cannot be identified. DTI, diffusion tensor imaging; PLIC, posterior limb of internal capsule; NA, not applicable
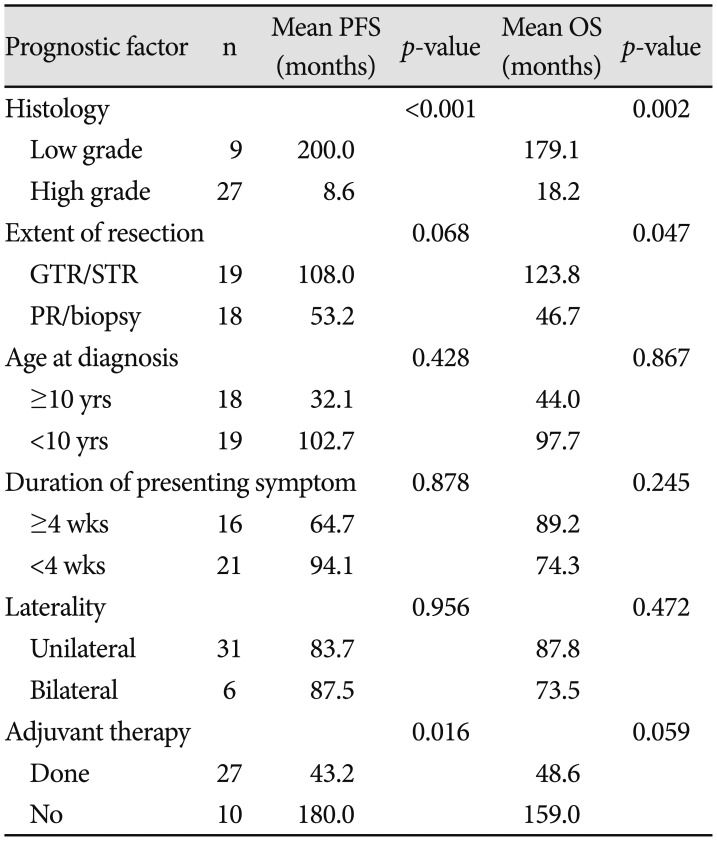
Table 3
Relevant prognostic factors to the mean PFS and mean OS




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download