Abstract
Acute acalculous cholecystitis (AAC) still remains one of the most elusive diagnoses and occurs in various conditions. Although AACs caused by viral infections are rare, various viruses have been revealed to cause AAC. Here we present a case in which a man suffered from AAC caused by a Hantaan virus infection. A 35-year-old man was referred to the emergency room for myalgia and fever that began 4 days ago. He suffered oliguria and abdominal pain for 2 days. At the time of his visit to the emergency room, he experienced a fever that spiked up to 38.3℃. An initial blood sample objectified the following pathologic results: white blood cells - 10260/µl; C-reactive protein – 6.76 mg/dl; total bilirubin – 1.7 mg/dl; AST – 90 IU/L; ALT – 233 IU/L. In the computed tomography, bilateral perirenal fluid collections and bilateral flexural effusion were shown and acute hepatopathy and cholecystopathy were also shown. Because there was no definite tenderness around the patient's right upper quadrant from physical examination and his cholecystopathy looked like it was from secondary change according to acute hepatopathy, we decided to perform conservative care without surgical treatment. The following day, in viral antibody test, Hantaan virus antibody was detected. After conservative management, the patient's condition improved and his laboratory findings were stable. The patient was discharged on the 10th day at the hospital stay without any symptoms. The Hantaan virus infection should be suspected as a causative agent of AAC, especially when there is abnormal liver function tests and abdominal pain.
Acute acalculous cholecystitis (AAC) is defined as the inflammation of the gallbladder wall in the absence of stones or sludge.1 It accounts for about 10% of acute cholecystitis.2 However, AAC still remains one of the most elusive diagnoses because it occurs in various conditions such as trauma, post-operation, burn, sepsis, fasting and so on.1 Viral infections also are one of the causes inducing ACC. Although AACs caused by viral infections are rare, various viruses have been revealed to cause AAC. Above them, AAC induced by hantavirus is extremely rare. Only few cases of AAC associated with hantavirus infection have been reported.345 Here we present a case in which a man suffered from AAC caused by a Hantaan virus, which is one of the subtypes of hantavirus.
A 35-year-old man was referred to the emergency room at the end of August for myalgia and fever that began 4 days ago [=post-onset of symptoms (POS) 0]. He suffered oliguria and abdominal pain for 2 days. At the time of his visit to the emergency room, he experienced a fever that spiked up to 38.3℃. Blood pressure was 112/57 mm Hg, respiratory rate was 18 breaths/min and weight of the patient was 81.0 kg. Numeric pain rating scale (NRS) was 3. However, abdominal palpation revealed no tenderness. An initial blood sample objectified the following pathologic results: white blood cells (WBC) – 10260/µl; C-reactive protein (CRP) – 6.76 mg/dl; total bilirubin – 1.7 mg/dl; aspartate aminotransferase (AST) – 90 IU/L; alanine aminotransferase (ALT) – 233 IU/L; and alkaline phosphatase (ALP) – 638 IU/L. Although specific analysis of urine analysis (specific gravity) was 1.030, the serum creatinine was still normal on POS 4, at 0.91 mg/dl. The chest radiograph was normal. The patient was admitted under r/o hemorrhagic fever with renal syndrome (HFRS). Korean hemorrhagic fever virus studies (R. tsutsugamushi Ab, Hantaan virus Ab and Leptospira Ab) were done.
One day later (POS 5), the abdominal pain was aggravated (NRS 5) and fever spiked up to 38.7℃. The patient gained weight about 3.5 kg and diuresis was only 690 ml/24 h. U/A S.G revealed 1.032. The blood sample objectified the following results: WBC – 11760/µl; CRP – 10.65 mg/dl; total bilirubin – 2.2 mg/dl; direct bilirubin – 1.8 mg/dl; AST – 43 IU/L; ALT; 157 IU/L; and ALP – 591 IU/L. In the computed tomography, bilateral perirenal fluid collections and bilateral pleural effusion were shown and pericholecystic fluid and subserosal edema with halo sign were also shown. (Fig. 1) Although abdominal pain was aggravated there was no definite tenderness around the patient's right upper quadrant from physical examination. Because his cholecystopathy looked like acute acalculous cholecystitis from secondary change according to acute hepatopathy, we decided to perform conservative care without surgical treatment. At night of the day (POS 5), Hantaan virus antibody was detected in the viral antibody test.
After conservative management, the patient's condition improved. Urine output increased rapidly to 3300 ml/24 hr on POS 8 and body weight decreased to 79.5 kg on POS 11. In the meantime, his laboratory findings were getting better. Comparative change of the laboratory findings are summarized in Table 1. The patient was discharged on POS 11 without any symptoms. The patient visited the outpatient department on POS 34 and the clinical examination was normal at that time.
Since the 1980s, when the Hantaan virus was reported, lots of studies about hantavirus were performed.6 In the present, various viruses apart from the hantaan virus are reported as a subtype of the hantavirus throughout the world. Hantavirus infection is presented with various symptoms such as fever, malaise and myalgia. It appears as two clinical syndromes: hemorrhagic fever with renal syndrome (HFRS) or hantavirus cardiopulmonary syndrome (HCPS).7
One of the mechanisms in the pathogenesis of hantavirus is plasma leakage. Hantavirus causes vascular permeability-based diseases and primarily infect endothelial cells.8 It leads to edematous swelling of organs such as kidney and lung. These are presented as pleural effusion and perirenal fluid collection.
Sometimes, this edematous change occurs in the gallbladder. A study reported that gallbladder wall thickening occurs in HFRS patients and it is related to the severity of the disease. Especially, more than 4 mm of gallbladder wall thickening is related to its severity.9 Although an ultrasonography was not performed in our patient, the computed tomography showed that his gallbladder wall thickness was more than 4 mm.
However, not every case with gallbladder wall thickening experiences acalculous cholecystitis. A case of acalculous cholecystitis related to hantavirus infection is rare. To the best of our knowledge, there are 2 case reports and 1 case series.345 By reviewing the previous cases, all of the patients underwent conservative care without cholecystectomy. AAC originated from viral disease tend to undergo conservative management. Especially, because most of ACC originated from viral disease, supportive care is recommended in children.10 In our case, the patient was discharged after conservative management.
One interesting finding in our case was that a Hantaan virus subtype caused ACC. The previous cases were reported from Europe and they were infected by the puumala virus subtype, a form of hantavirus. After Hantaan virus was first reported in the 1980s, about 30 subtypes of hantavirus have been reported.7 However, other than the puumala virus, there was no report that hantavirus induced ACC. Our case showed possibility that other subtypes of hantavirus could induce ACC.
In conclusion, this case relates the occurrence of ACC to the Hantaan virus subtype. As hantavirus causes plasma leakage leading to ACC, hantavirus induced ACC might be treated by supportive care without surgery. In addition, subtypes of hantavirus other than Hantaan or puumala subtypes could induce ACC. Further studies are needed.
Figures and Tables
Fig. 1
Computed tomographic findings of the patient. (A) White arrow: pericholecystic fluid and subserosal edema with halo sign. (B) White wedge: pleural effusion, white arrow: perirenal fluid collections.
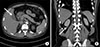
Table 1
Biological and clinical parameters of the patient

POS, post-onset of symptoms; WBC, white blood cell; Hb, hemoglobin; Plt, platelet; BUN, blood urea nitrogen; Cr, creatinine; CRP, c-reactive protein; Alb, albumin; LDH, lactate dehydrogenase; T. bil, total bilirubin; D. bil, direct bilirubin; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; U/A S.G, urinalysis specific gravity; BP, blood pressure; HR, heart rate; RR, respiratory rate; BT, body temperature; U/O, urine output
References
1. Barie PS, Eachempati SR. Acute acalculous cholecystitis. Gastroenterol Clin North Am. 2010; 39:343–357. x

2. Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol. 2010; 8:15–22.

3. Fröhlich R, Römmele U. [Acalculous cholecystitis in hantavirus infections]. Dtsch Med Wochenschr. 2013; 138:1255–1258. German.
4. Keyaerts E, Ghijsels E, Lemey P, Maes P, Zachée P, Daelemans R, et al. Plasma exchange-associated immunoglobulin m-negative hantavirus disease after a camping holiday in southern france. Clin Infect Dis. 2004; 38:1350–1356.

5. Nicolas JB. Acalculous cholecystitis associated with hemorrhagic fever with renal syndrome. Acta Clin Belg. 2015; 70:377–381.

6. Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. 1978. J Infect Dis. 2004; 190:1711–1721.
7. Avšič-Županc T, Saksida A, Korva M. Hantavirus infections. Clin Microbiol Infect. 2019; 21S:e6–e16.

8. Mackow ER, Gavrilovskaya IN. Hantavirus regulation of endothelial cell functions. Thromb Haemost. 2009; 102:1030–1041.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download