Abstract
Purpose
To investigate the blood pharmacokinetics and bio-distribution of DTPA-bis-amide (L3) Gd(III) complexes.
Materials and Methods
The pharmacokinetics and bio-distribution of Gd (L3)(H2O).nH2O were investigated in Sprague-Dawley rats after intravenous administration at a dose of 0.1 mmol Gd/kg. The Gd content in the blood, various tissues, and organs was determined by ICP-AES. Blood pharmacokinetic parameters were calculated using a two-compartment model.
Results
The half-lives of αphase and βphase Gd (L3)(H2O).nH2O were 2.286±0.11 min and 146.1±7.5 min, respectively. The bio-distribution properties reveal that the complex is mainly excreted by the renal pathway, and possibly excreted by the hepatobiliary route. The concentration ratio of Gd (III) was significantly higher in the liver and spleen than in other organs, and small amounts of Gd (III) ion were detected in the blood or other tissues of rats only after 7 days of intravenous administration.
Conclusion
The MRI contrast agent Gd (L3)(H2O).nH2O provides prolonged blood pool retention in the circulation and then clears rapidly with minimal accumulation of Gd(III) ions. The synthesis of gadolinium complexes with well-balanced lipophilicity and hydrophilicity shows promise for their further development as blood pool MRI contrast agents.
Magnetic resonance imaging (MRI) is a powerful, noninvasive diagnostic imaging modality that provides high-quality anatomic images and other physiologic data (1, 2). Advancement of the MRI technique has prompted the development of contrast agent, which is administered to the patient to enhance image contrast between normal and diseased tissue or to indicate the status of organ function or blood flow (3, 4). The development of new MRI contrast agents offers intriguing challenges for investigators in the chemical, physical, and biological sciences, including the design and synthesis of stable, non-toxic, and tissue-specific metal complexes and the understanding of their effect on relaxation behavior in solution and in tissues. MR image contrast is mainly generated by the relaxation differences of water protons in adjacent tissues. Gadolinium (III) ion has a large magnetic moment, which can alter the relaxation rate of surrounding protons to enhance image contrast (5). Gd (III) is known to possess the highest paramagnetism of all metal ions; to date, Gd (III) complexes incorporating macro cyclic or acyclic polyligands have been used widely as MRI contrast agents. These contrast agents catalytically shorten the relaxation time of nearby water molecules to enhance their contrast against background tissue on MR images.
The increasing use of MRI as a diagnostic imaging modality has prompted the development of efficient MRI contrast agents (6). The Gd-based MRI contrast agents currently available for clinical use can be classified into two types: anionic and neutral. The latter is preferred because it causes relatively low osmotic pressure in the body fluids after intravenous administration (7). Various contrast agents based on gadolinium ion complexes have been developed to enhance MRI signal intensity and to improve the contrast-to-noise ratio in regions of interest by shortening the longitudinal relaxation time (T1) of water protons. Current clinically used contrast agents, however, such as Gd(DTPA) and Gd(EOB-DTPA), are not suitable as blood pool contrast agents for the magnetic resonance angiography technique due to their short plasma half-lives caused by rapid elimination from the kidney and the liver, and/or the characteristic of free extravasations into background tissue, which rapidly reduces the contrast-to-noise ratio in blood vessels (8, 9).
For routine T1-weighted MRI examinations, it is desirable for a blood pool contrast agent to have the following characteristics: long plasma half-life, selective retention in the intravascular space to obtain a high contrast-to-noise ratio, and complete elimination from the blood after the MRI examination. Macromolecular MRI contrast agents based on albumin, polymers, and dendrimers have been developed as blood pool contrast agents, and have been shown to have long plasma half-lives with a high relaxivity, which is a parameter that represents the ability to act as a contrast agent. However, these macromolecular contrast agents have two limitations for clinical application: 1) the possibility of eliciting an immune response due to the use of proteins and large molecules, and/or 2) slow elimination from the body and subsequent metabolism, which could lead to the release of toxic by-products, including free Gd(III) (10).
To address these limitations and take advantage of the potential of macromolecular MRI contrast agents, low-molecular-weight gadolinium complexes have been chemically designed to bind noncovalently with substances such as plasma proteins. Based on the results of previous studies, it is considered that the balance between hydrophilicity and lipophilicity of a blood pool MRI contrast agent is the most important factor for controlling the plasma half-life and elimination from an organism (11).
It has been reported that the synthesis and characterization of Gd(III) complexes incorporating a new type of DTPA-bis-amides(L1-L3), functionalized by cyclohexyl carboxylates as the polar functional group, to achieve enhanced solubility in water (12, 13). The aim of this study was to perform an in vivo evaluation of blood circulation time and bio-distribution in various organs of Gd(L3)(H2O).nH2O as a contrast agent.
Gd(L3)(H2O).nH2O was prepared as previously described (6) (Fig. 1). Ketamine and xylazine were purchased from Ben Venue Labs (Janssen Korea Ltd) and Vedco Inc (Rompun, Hydrochloride, Bayer, US), respectively. Male Sprague-Dawley rats (250-350g) were used in the pharmacokinetics and Gd(III) tissue bio-distribution studies.
All animal studies were performed in compliance with guidelines set by the Institutional Animal Care and Use Committee of Kyungpook National University, Korea. A group of six rats were used for pharmacokinetic study of the contrast agent Gd(L3)(H2O).nH2O. Rats were anesthetized by intramuscular injection of a mixture of ketamine (45 mg/kg) and xylazine (6 mg/kg). The rats were tracheotomised, and the left carotid artery was catheterized for blood collection. The contrast agent Gd(L3)(H2O).nH2O was injected as a bolus through the femoral vein at a dose of 0.1 mmol Gd/kg. Blood samples were collected before and at 2, 6, 15, 30, 60, and 240 min after injection. The gadolinium content of the blood samples was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES, Thermo Jarrell Ash IRIS-AP, USA) after acid digestion (0.6 ml of HNO3, 0.3 ml of H2O2) in a microwave-based digestion system (Milestone MSL-1200, Sorisole, Italy).
A two-compartment pharmacokinetic model was used to simulate the concentration of the contrast agent in the blood after an intravenous bolus injection. WinNonLin (Pharsight Corporation) was used to fit the Gd(III) concentration data and calculate the pharmacokinetic parameters.
The bio-distribution or tissue accumulation of Gd (III) complexes is a critical parameter in determining their safety properties. In previous studies, tissue accumulation was determined at time points of 1, 3, 7, 24, and 72 hours, and 7 days after administration. A group of six male Sprague-Dawley rats was used for each agent to study the bio-distribution or Gd(III) accumulation in the major organs and tissues. The rats were injected at a dose of 0.1 mmol Gd/kg via the tail vein. The rats were sacrificed at various times post-injection and the organ and tissue samples (heart, liver, brain, spleen, and kidney) were collected and weighed. The gadolinium content of the samples was determined by ICP-AES (Thermo Jarrell Ash IRIS-AP) after acid digestion (0.6 ml of HNO3, 0.3 ml of H2O2) in a microwave-based digestion system (Milestone MSL-1200, Sorisole). The percentage of injected dose per organ/tissue was calculated to express the bio-distribution of the agents in the organs and tissues.
T1 measurements were carried out using an inversion recovery method with variable inversion times (TI) at 1.5 T (64 MHz). The MR images were acquired at 35 different TI values ranging from 50 to 1750 msec. T1 relaxation times were obtained from the non-linear least squares fit of the signal intensity measured at each TI value. For T2 measurement, the CPMG (Carr-Purcell-Meiboom-Gill) pulse sequence was adapted for multiple spin-echo measurements. Thirty-four images were acquired with 34 different echo time (TE) values ranging from 10 to 1900 msec. T2 relaxation times were obtained from the non-linear least squares fit of the mean pixel values for the multiple spin-echo measurements at each TE. In titration experiments with HSA (human serum albumin) (Sigma, St. Louis, MO), different amounts of the protein were added to a dilute aqueous solution of the complexes at PH 7.2 and the water proton relaxation times were measured after each addition, at 25 ℃. Relaxivity (r1) was then calculated as the inverse of relaxation time per mM. The determined relaxation times (T1 and T2) and relaxivities (r1 and r2) were then image-processed to create relaxation time maps and relaxivity maps, respectively.
The pharmacokinetic parameters of Gd(L3)(H2O).nH2O were determined in Sprague-Dawley rats. The kinetics of plasma concentrations for this compound relative to time are presented in Figure 2 (A, B). After 6 min, < 50% of the injected dose of Gd(L3)(H2O).nH2O was found in the blood. Thirty minutes later, the blood concentration had decreased to 11.47% of the injected dose. At the whole-animal level, 5.45, 2.95, and 1.1% of the dose was still detected at 1, 4, and 24 hours post injection, respectively. The pharmacokinetic parameters were calculated using a WinNonLin (Pharsight Corporation) fit to the blood concentration vs. time curves (Fig. 2B). The value of blood distribution (α=2.56 min) and elimination (β=141.6 min) phase of half-lives obtained for Gd(L3)(H2O).nH2O is clearly different from that reported for Gd-(DTPA-BMA) (14); the elimination of Gd(L3)(H2O).nH2O was significantly slower than that of Gd-(DTPA-BMA) (P<0.01) (14). The relatively long elimination half-life suggests that the agent is confined to the blood compartment, and thus could have important potential as a blood pool contrast agent.
The bio-distribution of the Gd(L3)(H2O).nH2O contrast agent was evaluated at several time periods after injection. The amount of Gd (III) in the different organs, as well as in the remaining body, was measured by ICP-AES. Fig. 3 shows temporal changes in extrinsic Gd (III) in the liver, kidney, spleen, heart, and brain from 15 min after the last injection until 7 days later, expressed as a percentage of the administrated dose. The great importance of liver deposition of the injected Gd (III) is apparent, with the kidney and spleen being of secondary importance; relatively little deposition occurred in any of the other analyzed organs. Fifteen minutes after the last injection, the concentration of Gd (III) was much higher in the liver than in other organs (Fig. 4). By 3-24 hours post injection, over 8% of the dose was retained in the liver, after which a rapid decrease in Gd (III) concentration was observed (Fig. 3). Thus, the liver eliminated all of the injected Gd (III) between Days 1 and 7, indicating a highly efficient excretory mechanism that presumably involves bile.
Many protein-binding complexes are believed to interact with hepatocytes, and although the mechanism of interaction is not completely understood, the dissociated chelate is removed from circulation by the hepatobiliary route. In the untreated rats, a low Gd (III) concentration was detected in the liver. In contrast, the high Gd (III) concentration measured in the kidney suggests that Gd(L3)(H2O).nH2O has a renal route of excretion. The accumulation of Gd (III) in the spleen is slightly less than that in the liver, possibly due to internalization of polymeric conjugates by macrophages. The concentration of Gd (III) in the heart and brain gradually decreased over time (Fig. 3). Figure 3 also shows gadolinium concentrations in several tissues (expressed as percentage of dose) after 7 days of 0.1 mmol Gd/kg Gd(L3)(H2O).nH2O IV administration in five rats. Low concentrations of gadolinium were found in the liver (0.76%), spleen (0.88%), kidneys (0.05%), brain (0.009%), and heart (0.01%).
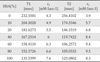
The noncovalent binding of Gd (III) complexes with HSA can be conveniently studied by relaxometric methods using a low-resolution NMR spectrometer operating at a fixed frequency. Titration of a dilute aqueous solution of the complex with the protein causes an increase in the relaxation rate when a binding interaction occurs as a result of an increase in the reorientational correlation time of the macromolecular adduct. Analysis of the data according to the proton relaxation enhancement method yields good estimates of the association constant, KA, and of the relaxivity (r1) of the bound complex. All the investigated complexes show some degree of interaction with HSA (Table 1).
As mentioned above, a high protein-binding ability is required to achieve a long plasma half-life and subsequent selective retention of the blood pool contrast agent in the intravascular space. Therefore, the protein-binding ability of the Gd(L3)(H2O).nH2O compound was assessed by measuring its longitudinal relaxivity r1 value in the presence of proteins (HSA). It is known that the binding of contrast agents to large molecules such as HSA enhances the efficacy of the electronuclear interaction between the gadolinium ion and water protons due to a decrease in molecular tumbling of the protein-bound agent and an increase in the correlation time between them (4), leading in turn to shortened T1 and bright blood vessels on MRI images (2, 14). For this reason, an experimental observation of an increased r1 value of Gd(L3)(H2O).nH2O indicates binding between the contrast agent and HSA (Fig. 5).
The following features are desired in a blood pool contrast agent to improve the contrast-to-noise ratio of the blood vessels of interest in MR images: 1. long plasma half-life, 2. retention of the contrast agent in the intravascular space (minimizing leakage to extracellular space), and 3. complete elimination of Gd(III) from the body after the MRI examination. To meet these requirements, it is necessary to control the balance between the lipophilicity and hydrophilicity of the contrast agent to realize appropriate in vivo functionality and drug kinetics by avoiding excessively rapid excretion from the kidney and liver.
It has been reported that Gd-complexes with a new DTPA-bis-amides formulation (12, 13) have the following properties: much higher r1 relaxivity than Omniscan; increased stability under physiological conditions, as indicated by their thermodynamic stability constants, conditional stability constants; and cytotoxicity as low as that for Omniscan, as revealed by MTT assay performed on these complexes in the concentration range required to obtain intense enhancement in in vivo MRI studies.
Lipophilicity is a critical parameter in promoting noncovalent binding of the contrast agent to the lipid binding sites of HSA, which is known as a lipid transporter and which exists in high concentrations in the blood (11). In the present study, we systemically evaluated the pharmacokinetics and bio-distribution of Gd(L3)(H2O).nH2O in the major organs and tissues of rats, and examined its ability to bind to albumin.
The observed plasma half-life of Gd(L3)(H2O).nH2O was 141.6 min in healthy rats, which is longer than the value of 17.6 min for the reference compound Magnevist. The longer plasma half-life of Gd(L3)(H2O).nH2O is attributed to appropriate control of the balance between lipophilicity and hydrophilicity using aromatic groups and sulfonic acids, which suppress rapid excretion from the kidney and the liver by enabling the contrast agents to bind with plasma proteins.
The design of selective contrast agents for blood pool imaging (e.g., MS-325, EPIX Medical, Cambridge, MA) or liver imaging (Gd-EOB-DTPA, Primovist, Schering AG) is an area of active research (15, 16). An interest interesting feature of the bio-distribution of Gd(L3)(H2O).nH2O (Fig. 4) is its high selectivity for the liver over the blood pool. The results of the present study indicate that chelated hydrophobicity is the driver for liver selectivity.
Contrast agent can interact by noncovalently binding to proteins present in blood plasma. The most important protein in human plasma is serum albumin (HSA), which constitutes 4-4.5% of plasma. This large globular protein binds to a variety of molecules, such as drugs, metabolites, and fatty acids (17). Noncovalent interaction of MRI contrast agent with HSA reduces the molecular tumbling rate of the contrast agent, thereby increasing its relaxivity. Figure 5 shows the longitudinal proton relaxation rate in solutions containing different percentages of HSA, as a function of Gd(L3)(H2O).nH2O concentration. Binding to albumin reduces the amount of free drug that can extravasate from the blood pool into the nonvascular space and provides selective enhancement of the vascular relaxation rate. Binding also reduces the fraction of free chelate available for glomerular filtration by the kidneys. This slows the renal excretion rate, which in turn extends the half-life in the blood and increases the time available for imaging.
The present bio-distribution analysis revealed the accumulation of contrast agent in specific organs. In good agreement with its slower elimination, the bio-distribution of Gd(L3)(H2O).nH2O in rats after 15 min of intravenous administration (Fig. 4) indicates significantly higher concentrations in the kidney and liver than in the other organs analyzed. The Gd (III) concentration measured in the kidney suggests that Gd(L3)(H2O).nH2O has a renal route of excretion, while the relatively high Gd (III) concentration detected in the liver could be related to its lipophilic properties, leading to a possible hepatobiliary route of excretion. In fact, many albumin-binding complexes are believed to interact with hepatocytes, and although the mechanism of interaction is not completely understood, the dissociated chelate is removed from circulation by the hepatobiliary route. As shown in Figure 4, there was little difference in the Gd (III) bio-distribution in the brain from 15 min to 3 days. None of the complexes passed through the blood-brain barrier, as expected for high-molecular-weight and/or poorly lipophilic complexes. In fact, several authors estimate the molecular cut-off weight for brain extraction to be 400-600 Da for substances with log P >2 (18, 19).
In the present study, a new type of contrast agent, bearing two DTPA units able to bind two paramagnetic centers, was synthesized, characterized, preclinically evaluated, and tested for protein binding avidity. Data from relaxivity measurement analysis proved that this dimeric complex has high relaxivity, which increases in a solution of HSA because of the relatively strong interaction with this blood protein. Interaction with HSA also results in longer elimination half-life and better confinement to the vascular space, as shown in pharmacokinetic evaluation.
In conclusion, our present in vivo study suggests that Gd(L3)(H2O).nH2O has potential as a blood pool and potential organ-specific MRI contrast agent.
Figures and Tables
Fig. 2
a. Blood concentration-time curves of Gd in rats exposed to Gd (L3)(H2O).nH2O. Data are presented as absolute values of plasma concentration and percentages of IV dose. b. Schematic presentation of pharmacokinetic indices in Gd (L3)(H2O).nH2O. Mean plasma concentration-time profiles of Gd.

Fig. 3
Time course of distribution of Gd to tissues at various times after the last injection of the L3. Error bars are average deviations of values for pairs of rats.

Fig. 4
Bio-distribution of gadolinium in rats 15 min after intravenous injection of Gd(L3)(H2O).nH2O at a dose of 0.1 mmol Gd/kg. Data presented as mean ± SD.

Acknowledgments
This work was supported by NRF through the Basic Science Research Program (2011-0015353).
References
1. Mansfield P. Nmr imaging in biomedicine: Supplement 2 advances in magnetic resonance. 1982. Access Online via Elsevier.
2. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the MRI single-subject brain. Neuroimage. 2002; 15:273–289.
3. Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: Mathematical approach and statistical analysis. Magn Reson Med. 1996; 36:715–725.
4. Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.
5. Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium (iii) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999; 99:2293–2352.
6. Jacques V, Desreux JF. New classes of MRI contrast agents. Contrast agents i. Springer;2002. p. 123–164.
7. Aime S, Botta M, Terreno E. Gd (iii)-based contrast agents for MRI. Adv Inorg Chem. 2005; 57:173–237.
8. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997; 7:91–101.
9. Hamm B, Staks T, Muühler A, et al. Phase i clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: Safety, pharmacokinetics, and MR imaging. Radiology. 1995; 195:785–792.
10. Kobayashi H, Kawamoto S, Jo SK, Bryant HL Jr, Brechbiel MW, Star RA. Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjug Chem. 2003; 14:388–394.
11. Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006; 35:512–523.
12. Dutta S, Park JA, Jung JC, Chang Y, Kim TJ. Gd-complexes of DTPA-bis (amide) conjugates of tranexamic acid and its esters with high relaxivity and stability for magnetic resonance imaging. Dalton Trans. 2008; 28:2199–2206.
13. Gu S, Kim HK, Lee GH, Kang BS, Chang Y, Kim TJ. Gd-complexes of 1, 4, 7, 10-tetraazacyclododecane-n, n', n'', n'''-1, 4, 7, 10-tetraacetic acid (DOTA) conjugates of tranexamates as a new class of blood-pool magnetic resonance imaging contrast agents. J Med Chem. 2011; 54:143–152.
14. Wedeking P, Kumar K, Tweedle M. Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging. 1992; 10:641–648.
15. Parmelee DJ, Walovitch RC, Ouellet HS, Lauffer RB. Preclinical evaluation of the pharmacokinetics, biodistribution, and elimination of MS-325, a blood pool agent for magnetic resonance imaging. Invest Radiol. 1997; 32:741–747.
16. Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses gd-eob-dtpa (gadoxetic acid) with standard gddtpa. Invest Radiol. 2009; 44:305–310.
17. Fasano M, Curry S, Terreno E, et al. The extraordinary ligand binding properties of human serum albumin. IUBMB life. 2005; 57:787–796.
18. Samiotaki G, Vlachos F, Tung YS, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using mri. Magn Reson Med. 2012; 67:769–777.
19. Borlongan CV, Emerich DF. Facilitation of drug entry into the cns via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, cereport. Brain Res Bull. 2003; 60:297–306.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download