Abstract
Purpose
The purpose of this study was to setup a concuurent transcranial magnetic stimulation (TMS)-functional MRI (fMRI) system for understanding causality of the functional brain network.
Materials and Methods
We manufactured a TMS coil holder using nonmagnetic polyether ether ketone (PEEK). We simulated magnetic field distributions in the MR scanner according to TMS coil positions and angles. To minimize image distortions caused by TMS application, we controlled fMRI acquisition and TMS sequences to trigger TMS during inter-volume intervals.
Results
Simulation showed that the magnetic field below the center of the coil was dramatically decreased with distance. Through the MR phantom study, we confirmed that TMS application around inter-volume acquisition time = 100 miliseconds reduced imaging distortion. Finally, the applicability of the concurrent TMS-fMRI was tested in preliminary studies with a healthy subject conducting a motor task within TMS-fMRI and passive motor movement induced by TMS in fMRI.
Figures and Tables
Fig. 1
MR compatible figure-eight coil
a. Magnetic field induced by transcranial magnetic stimulation (TMS) figure-eight coil. Red area is focal point of induced magnetic field.
b. Specification of the figure-eight coil
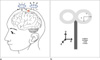
Fig. 2
TMS coil holder.
a. 3D graphic design of the prototype.
b. TMS coil holder made from PEEK and polymer composites.

Fig. 3
(a) Task paradigm and (b) triggering system.
a. The visual cue-based voluntary hand movement and concurrent TMS pulse. These voluntary hand movement and single shot TMS pulses are not periodic but randomly distributed.
b. A scheme of a pilot study of concurrent TMS-fMRI experiment using our own TMS coil holder. Triggering system is based on TR signal from the MR scanner and E-prime software for minimizing TMS-induced artifact during interleaved experiment.

Fig. 4
(a) MR compatible coil holder setup and (b) neuronavigation system.
a. MR-compatible holder setup for the MR phantom and healthy subject tasks.
b. Neuronavigator (Brainsight2) guides the motor region. Activation map obtained from a simple motor task was overlaid on top of the individual's own structural image.
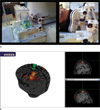
Fig. 5
Distribution of magnetic field induced by figure-eight coil and depending on distance from the coil: (a) at z=1 mm; (b) at z=10 mm; (c) at z=20 mm; (d) at z=30 mm. First and second column show the strength of magnetic field by color on x-y plane. The third column presents the magnetic field induced by every coil inside of the figure-eight coil Red spot is the most focused area.

Fig. 6
Distributions of TMS induced magnetic field - temporal dependency.
The distribution of magnetic field induced by TMS coil (red curve) and summation of MR magnetic field (arrows in the yellow box): (a) TMS coil is perpendicular to the MR magnetic field; (b) -30 degree rotation; (c) +30 degree rotation.
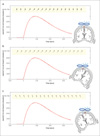
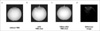
Fig. 7
MR phantom EPI data.
(a) without TMS coil, (b) with TMS shot, (c) 100ms after TMS shot and (d) difference between image (b) and (c).
White arrows indicate positions of TMS coil. There is no image distortion in the absence of the TMS coil. A single shot TMS during scanning distorts the B0 field and disturbs EPI. This disturbance persists for at least 100 ms. The bottom image was acquired with optimized MR sequence and triggering system. There is no image distortion in the absence of the TMS coil.
References
1. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985; 1(8437):1106–1107.
2. Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007; 9:527–565.
3. Wassermann EM, McShane L, Hallett M. ScienceDirect - Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section: Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992; 85:1–8.
4. Nardone R, De Blasi P, Bergmann J, et al. Theta burst stimulation of dorsolateral prefrontal cortex modulates pathological language switching: A case report. Neurosci Lett. 2011; 487:378–382.
5. Funke K, Benali A. Modulation of cortical inhibition by rTMS-findings obtained from animal models. J Physiol. 2011; 589:4423–4435.
6. Di Lazzaro V, Dileone M, Pilato F, et al. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. 2011; 105:2150–2156.
7. Speer AM, Kimbrell TA, Wassermann EM, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000; 48:1133–1141.
8. Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005; 45:201–206.
9. Stagg CJ, Wylezinska M. Neurochemical Effects of Theta Burst Stimulation as Assessed by Magnetic Resonance Spectroscopy. J Neurophysiol. 2009; 101:2872–2877.
10. Huang YZ, Rothwell JC, Chen RS, Lu CS, Chuang WL. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2011; 122:1011–1018.
11. Lipton RB, Pearlman SH. Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics. 2010; 7:204–212.
12. Pennisi G, Alagona G, Rapisarda G, et al. Transcranial magnetic stimulation after pure motor stroke. Clin Neurophysiol. 2002; 113:1536–1543.
13. Najib U, Bashir S, Edwards D, Rotenberg A, Pascual-Leone A. Transcranial brain stimulation: clinical applications and future directions. Neurosurg Clin N Am. 2011; 22:233–251.
14. Kamarajan C, Porjesz B, Jones KA, et al. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005; 69:353–373.
15. Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004; 19:1950–1962.
16. Bohning DE, Denslow S, Bohning PA, Walker JA. ScienceDirect.com - Clinical Neurophysiology - A TMS coil positioning/holding system for MR image-guided TMS interleaved with fMRI. Clin Neurophysiol. 2003; 114:2210–2219.
17. Driver J, Blankenburg F, Bestmann S, Ruff CC. New approaches to the study of human brain networks underlying spatial attention and related processes. Exp Brain Res. 2010; 206:153–162.
18. Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci Biobehav Rev. 2011; 35:516–536.
19. Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: from "virtual lesions" to functional-network accounts of cognition. Cortex. 2009; 45:1043–1049.
20. Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010; 22:219–232.
21. Moisa M, Pohmann R, Ewald L, Thielscher A. New coil positioning method for interleaved transcranial magnetic stimulation (TMS)/functional MRI (fMRI) and its validation in a motor cortex study. J Magn Reson Imaging. 2009; 29:189–197.
22. MATLAB(The MathWorks, Inc.) [Internet]. cited 2013 Jun 18. Available from: http://www.mathworks.com/.
23. E-prime(Psychology software tools, INC) [Internet]. cited 2013 Jun 18. Available from: http://www.pstnet.com/eprime.cfm.
24. Brainsight2(Rogue Research Inc) [Internet]. cited 2013 Jun 18. Available from: https://www.rogue-research.com/.
25. Statistic Parametric Mapping (SPM8) [Internet]. cited 2013 Jun 18. Available from: http://www.fil.ion.ucl.ac.uk/spm/.
26. Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011; 1:13–36.
27. Smith JF, Pillai A, Chen K, Horwitz B. Effective connectivity modeling for fMRI: six issues and possible solutions using linear dynamic systems. Front Syst Neurosci. 2012; 5:104.
28. Heinen K, Ruff CC, Bjoertomt O, et al. Concurrent TMS-fMRI reveals dynamic interhemispheric influences of the right parietal cortex during exogenously cued visuospatial attention. Eur J Neurosci. 2011; 33:991–1000.
29. Peters JC, Reithler J, Schuhmann T, et al. On the feasibility of concurrent human TMS-EEG-fMRI measurements. J Neurophysiol. 2013; 109:1214–1227.
30. Ilmoniemi RJ, Kicić D. Methodology for combined TMS and EEG. Brain Topogr. 2010; 22:233–248.
31. Bestmann S, Oliviero A, Voss M, et al. Cortical correlates of TMS-induced phantom hand movements revealed with concurrent TMS-fMRI. Neuropsychologia. 2006; 44:2959–2971.
32. Li B, Daunizeau J, Stephan KE, Penny W, Hu D, Friston K. Generalised filtering and stochastic DCM for fMRI. Neuroimage. 2011; 58:442–457.
33. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009; 120:2008–2039.
34. Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005; 1:e42.
35. Behrens TE, Sporns O. Human connectomics. Curr Opin Neurobiol. 2012; 22:144–153. Epub 2011 Sep 9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download