Abstract
Purpose
To evaluate and compare the accuracy of magnetic resonance imaging (MRI) and ultrasound (US) for detection and estimation of invasion depth of colorectal carcinoma (CRC) by correlation with histopathologic findings in vitro, and to find out the best MR pulse sequence for accurate delineation of tumor from surrounding normal tissue.
Materials and Methods
Resected specimens of CRC from 45 patients were examined about tumor detectability and invasion depth of US using high frequency (5-17 MHz) linear transducer in a tube filled with normal saline and MRI in a 8-channel quadrate head coil. The institutional review board approved this study and informed consent was waived. MRI with seven pulse sequences of in- and out-of-phases gradient echo T1 weighted images, fast spin echo T2 weighted image and its fat suppression image, fast imaging employing steady-state acquisition (FIESTA) and its fat suppression image, and diffusion weighted image (DWI) were performed. In each case, both imaging findings of MRI and US were evaluated independently for detection and estimation of invasion depth of tumor by consensus of two radiologists and were compared about diagnostic accuracy according to the histopathologic findings as reference standard. Seven MR pulse sequences were evaluated on the point of accurate delineation of tumor from surrounding normal tissue in each specimen.
Results
In specimens of CRC, both imaging modalities of MRI (91.1%) and US (86.7%) showed relatively high diagnostic accuracy to detect tumor and evaluate invasion depth of tumor. In early CRC, diagnostic accuracy of US was 87.5% and that of MRI was 75.0%. There was no statistically significant difference between two imaging modalities (p > 0.05). The best pulse sequence among seven MR sequences for accurate delineation of tumor from surrounding normal tissue in each specimen of CRC was fast spin echo T2 weighted image.
Figures and Tables
Fig. 1
Rectal cancer (T1, polypoid type).
a-b, g. T1 in- and out-of-phases and DWI don't show exact layer of tumor involvement.
c-f. FSE T2, FSE T2 fat-suppression, FIESTA and FIESTA fat-suppression show tumor involvement of submucosal layer with preserved proper muscle layer (arrows).
h. US shows hypoechoic tumor involvement (arrow) of echogenic submucosa with preserved hypoechoic proper muscle layer.
i. Histopathology of the tumor (H & E, ×10) is confined to submucosa.
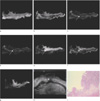
Fig. 2
Rectal cancer (T2, ulcerative type).
a, e. T1in-phase and FIESTA don't show exact layer of tumor involvement.
b-c, d, f-g. T1 out-of-phase, FSE T2, FSE T2 fat-suppression, FIESTA fat-suppression and DWI show tumor involvement of submucosal layer with disruption of proper muscle layer (arrows).
h. US shows hypoechoic tumor involvement of echogenic submucosa with just thickened inner hypoechoic muscle layer (arrow).
i. Histopathology of the tumor (H & E, ×10) is invasion to proper muscle layer.
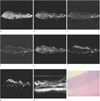
Fig. 3
Sigmoid colon cancer (T3, ulcerofungating type).
a-b, g. T1 in- and out-of-phases and DWI don't show exact layer of tumor involvement.
c-f. FSE T2, FSE T2 fat-suppression, FIESTA and FIESTA fat-suppression show tumor infiltration into pericolic fat tissue (arrows).
h. US shows hypoechoic tumor infiltration into echogenic pericolonic fat tissue (arrow).
i. Histopathology of the tumor (H & E, ×10) is infiltration into pericolic fat tissue.
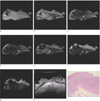
Fig. 4
Sigmoid colon cancer (T3, ulceroinfiltrative type).
a-c, e-f. T1 in- and out-of-phases, FSE T2, FIESTA and FIESTA fat-suppression show infiltrative tumor invasion to pericolic fat tissue (arrows).
d, g. FSE T2 fat-suppression and DWI don't show exact layer of tumor involvement.
h. US shows ill-defined hypoechoic tumor infiltration into echogenic pericolic fat tissue (arrow).
i. Histopathology of the tumor (H & E, ×10) is invasion into pericolic fat tissue.
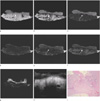
Table 2
Ratio of Satisfying Imaging among Seven MR pulse Sequences

Note.-T1-in : gradient echo T1 weighted in-phase image
T1-out : gradient echo T1 weighted out-of-phase image
FSE T2 : fast spin echo heavily T2 weighted image
FSE T2 fat-sup : fast spin echo heavily T2 weighted fat suppression image
FIESTA : fast imaging employing steady-state acquisition image
FIESTA fat-sup : fast imaging employing steady-state acquisition with fat suppression image
DWI : diffusion weighted image (b = 1000)
References
1. Stastics Korea. 2009 causes of death stastics. http://www.kostat.go.kr.
2. Visser BC, Varma MG, Welton ML. Local therapy for rectal cancer. Surg Oncol. 2001; 10:61–69.
3. Hundt W, Braunschweig R, Reiser M. Evaluation of spiral CT in staging of colon and rectum carcinoma. Eur Radiol. 1999; 9:78–84.
4. Horton KM, Abrams RA, Fishman EK. Spiral CT of colon cancer: imaging features and role in management. Radiographics. 2000; 20:419–430.
5. Kudo S, Kashida H, Nakajima T, Tamura S, Nakajo K. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg. 1997; 21:694–701.
6. Okabe S, Arai T, Maruyama S, Murase N, Tsubaki M, Endo M. A clinicopathological investigation on superficial early invasive carcinomas of the colon and rectum. Surg Today. 1998; 28:687–695.
7. Akin O, Nessar G, Agildere AM, Aydog G. Preoperative local staging of rectal cancer with endorectal MR imaging: comparison with histopathologic findings. Clin Imaging. 2004; 28:432–438.
8. Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004; 232:335–346.
9. Maldjian C, Smith R, Kilger A, Schnall M, Ginsberg G, Kochman M. Endorectal surface coil MR imaging as a staging technique for rectal carcinoma: a comparison study to rectal endosonography. Abdom Imaging. 2000; 25:75–80.
10. Gualdi GF, Casciani E, Guadalaxara A, d'Orta C, Polettini E, Pappalardo G. Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings. Dis Colon Rectum. 2000; 43:338–345.
11. Reynolds JV, Joyce WP, Dolan J, Sheahan K, Hyland JM. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg. 1996; 83:1112–1115.
12. Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001; 357:497–504.
13. Burton S, Brown G, Daniels IR, et al. MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer. 2006; 94:351–357.
14. Lahaye MJ, Engelen SM, Nelemans PJ, et al. Imaging for predicting the risk factors the circumferential resection margin and nodal disease of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005; 26:259–268.
15. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging-a meta-analysis. Radiology. 2004; 232:773–783.
16. Otchy D, Hyman NH, Simmang C, et al. Practice parameters for colon cancer. Dis Colon Rectum. 2004; 47:1269–1284.
17. Thickman DI, Kundel HL, Wolf G. Nuclear magnetic resonance characteristics of fresh and fixed tissue: the effect of elapsed time. Radiology. 1983; 148:183–185.
18. de Lange EE, Fechner RE, Edge SB, Spaulding CA. Preoperative staging of rectal carcinoma with MR imaging: surgical and histopathologic correlation. Radiology. 1990; 176:623–628.
19. Beets-Tan RG, Lettinga T, Beets GL. Pre-operative imaging of rectal cancer and its impact on surgical performance and treatment outcome. Eur J Surg Oncol. 2005; 31:681–688.
20. Yamada I, Okabe S, Enomoto M, et al. Colorectal carcinoma: in vitro evaluation with high-spatial-resolution 3D constructive interference in steady-state MR imaging. Radiology. 2008; 246:444–453.
21. Akbari RP, Wong WD. Endorectal ultrasound and the preoperative staging of rectal cancer. Scand J Surg. 2003; 92:25–33.
22. Blomqvist L, Machado M, Rubio C, et al. Rectal tumour staging: MR imaging using pelvic phased-array and endorectal coils vs endoscopic ultrasonography. Eur Radiol. 2000; 10:653–660.
23. Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009; 253:116–125.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download