Abstract
Cerebral fat embolism (CFE) is a rare, albeit potentially lethal complication of long-bone fractures. All trauma patients who are initially lucid and subsequently experience mental status deterioration should undergo immediate evaluation of possible CFE. In the present case, magnetic resonance imaging (MRI) was the most sensitive technique for the diagnosis of CFE, particularly the use of diffusion-weighted images (DWI). The authors present this case to report a pathophysiology-based interpretation of the MR characteristics and treatment of CFE.
Cerebral fat embolism (CFE) is a rare, albeit serious complication of long-bone fractures. CFE results in variable neurological disability, often associated with a significant recovery time. Magnetic resonance imaging (MRI), particulary diffusion-weighted imaging (DWI), is the most sensitive method to establish a diagnosis of CFE, with typical findings of multiple punctate high-intensity lesions within the brain parenchyma. The pathophysiology of CFE remains unclear; However, it is considered a direct expression of small vessel occlusion by fat droplets. The authors present a patient with abrupt and profound neurological deterioration after long-bone fractures for which early supportive treatment with rapid diagnostic confirmation using brain MRI led to a significant recovery.
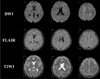
A healthy 16-year-old male was admitted to our hospital with an uncomplicated closed left femur and tibiofibular shaft fractures. He remained conscious throughout, with a Glasgow Coma Scale (GCS) score of 15. Computed tomography (CT) of the head and cervical spine, which was performed to exclude the presence of occult injury was normal on admission. The fractures were to be treated the following day with open reduction and intramedullary nail fixation. That night, however, 14 hours after the accident, the patient lost consciousness. A brain CT scan performed two hours later, revealed normal findings. MRI was performed eight hours later using DWI, T2-weighted imaging (T2WI), and fluid-attenuated inversion recovery (FLAIR) imaging, which showed the presence of multiple high-intensity lesions in the cerebellum, both basal ganglia, both thalami, the centrum semiovale, and the subcortical white matter of both hemispheres (Fig. 1).
Multiple lesions were prominent in the subcortical white matter. A CFE was suspected, and the workup included transesophageal echocardiography, which demonstrated the absence of patent foramen ovale. No pulmonary symptoms were present, and the arterial blood gas analysis (ABGA) was normal. Chest CT angiography revealed no evidence of pulmonary embolism. No cutaneous petechial hemorrhage was observed.
The patient received anticoagulation therapy to prevent the progression of thrombosis. This therapy consisted of subcutaneous low-molecular-weight heparin injections (50 antifactor Xa IU/kg) for five days.
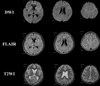
A follow-up MRI was performed two weeks after the onset of the cognitive impairment. The T2WI and DWI showed an interval decrease in the number and extent of hyperintensities in the entire brain (Fig. 2). The patient recovered marked neurologic functioning, but suffered mild recent memory impairment.
Repeat cerebral MRI was performed one month after the initial MRI examination. DWI and T2WI demonstrated a nearly resolved hyperintensity lesion that was noted on the previous MR image (Fig. 1C). The patient was discharged without neurological abnormalities.
Fat embolism syndrome (FES) is a clinical constellation of acute respiratory distress with hypoxia, cutaneous petechial hemorrhage, and variable neurological dysfunction. Its incidence is 0.5-3.5% of displaced long-bone fractures (1). However, CFE is far less common than FES, as most fat emboli would be captured by the pulmonary capillaries. Most CFE occur with right-to-left cardiac shunt, such as patent foramen ovale, but may also occur without any cardiac abnormality. In cases of right-to-left shunt, fat emboli can reach the systemic circulation simply by bypassing the pulmonary vasculature. Without right-to-left shunt, however, it is thought that the fat emboli may reach the systemic circulation by penetrating the pulmonary capillaries via the process of fat deformation (2). In the present case, the patient had no pulmonary symptoms and no patent foramen ovale was identified. The minimal pulmonary dysfunction that occurred during the ictus was not sufficient to cause global cerebral hypoxia; therefore, different explanation must be proposed. Echocardiography failed to reveal any cardiac abnormalities, such as patent foramen ovale. Chest CT angiography also demonstrated no pulmonary capillary compromise. Although CFE may occur in the absence of right-to-left shunt, cardiac and pulmonary evaluation is the mandatory proactive measure of the advent of pulmonary embolism.
Clinical manifestations of CFE vary considerably, from subclinical presentation to confusion to coma and seizures. In most cases, an asymptomatic latent period of about 12~48 hours precedes the clinical manifestations. The fulminant form presents as acute cor pulmonale, respiratory failure, or CFE leading to death within a few hours of the injury. There are several reports of isolated neurological abnormality and even death after CFE (3, 4). Takahashi et al. (5), reported that the GCS score of patients is closely related to the number of white matter lesions on MR images. In most cases, neurological dysfunction is recovered gradually over days or weeks. In the present case, the patient's neurological recovery was accompanied by a decrease in the number and extent of hyperintensity lesions, based on follow-up T2W MRI.
The pathogenesis of CFE has not been clearly elucidated. Regarding the source of fat emboli, most authors believe that these fat droplets originate at the site of the fracture and are conveyed to the lungs via the venous circulation. Fat emboli may pass into the systemic circulation either via cardiac or intrapulmonary right-to-left shunts or directly through the pulmonary capillary bed (3). An experimental study demonstrated that fat globules with a diameter of less than 5 µm can traverse the pulmonary microvasculature (6). This could explain CFE without pulmonary manifestation or right-to-left shunt.
The etiology of the neurological dysfunction induced by CFE remains largely unexplained. Erdem et al. (7) suggested that direct occlusion of microvasculature by fat emboli causes ischemic insults, which lead to focal abnormalities on MRI. In the present case, DWI MRI obtained 10 hours after the ictus revealed the presence of multiple small restrictions. The disruption of endothelial walls by chemical mediators explains the enhancement of multiple punctate lesions on contrast-enhanced T1-weighted images (T1WI) (2). However, in the present case, enhancement was not demonstrated in contrast-enhanced T1WI.
The CT findings of CFE were described previously (8). Most often, brain CT results are normal, as was observed in the present case. CT scans may show diffuse edema with scattered low-density areas as well as hemorrhage in some cases. MR findings in CFE have also been described previously in several reports (3, 5, 9). These findings include tiny, scattered, and hyperintense lesions on T2WI and FLAIR images. These lesions are typically predominant in the periventricular, subcortical, and deep white matter, as well as in the deep gray matter, including the corpus callosum. Generally, the gray matter is notably spared or only minimally involved. If the CFE is the expression of infarction, the preferential localization of the lesion in the cerebral white matter remains a paradox as usually this is an area where emboli are less frequent. The sparing of the gray matter could be explained by its rich vascularity from the sufficient anastomotic channel to protect itself against the ischemic effects of microemboli (6).
The number and size of lesions is variable but correlates with the degree of neurological disability, as measured by the GCS (5). MR images change with time after CFE. In the present case, the MR image obtained 14 hours after the ictus demonstrated the presence of multiple small, and scattered T2WI and DWI hyperintensity lesions, predominantly in the subcortical white matter. However, these diffuse white matter signal intensity abnormalities were normalized on repeat MRI performed two and four weeks after the ictus. This suggest strongly that the pathophysiology of CFE does not stem solely from the cytotoxic edema as a result of arteriolar occlusion by fat droplets, but could also result from the generalized and transient neurotoxic effect of local free fatty acids (2).
There is no specific therapy for CFE. The treatment is, therefore, supportive. Early diagnosis and adequate symptomatic treatment are of paramount importance. Supportive care includes maintenance of adequate oxygenation and ventilation, stable hemodynamics, hydration, and prophylaxis of deep vein thrombosis.
Because an intravasated fat droplet from the fat marrow of a fractured bone is the mastermind of CFE, interception of that fat droplet via stabilization of the fracture is one of the most important steps of the treatment of CFE. Early immobilization of fractures reduces the incidence of FES; the risk is further reduced by operative correction, rather than conservative management.
The main pathological findings of CFE includes microvascular occlusion and secondary activation of fibrin and platelet aggregation. Therefore, treatment should be directed toward the prevention of the progression of thrombosis. Anticoagulation allows the fibrinolytic system to function unopposed, ultimately decreasing the thromboembolic burden. In the present case, the patient was administered low-molecular-weight heparin.
Although the administration of corticosteroids with the aim of reducing the inflammatory response has been reported as an effective treatment in several trials (10), this remains controversial and limited evidence exists to support this treatment. However, given the pathogenesis of CFE, steroid therapy may diminish the inflammatory cascade and eventually reduce vasogenic edema.
Mortality from FES is estimated at 5-15% overall; however, most patients recover fully (1). There are few reports about the outcome of CFE. Some case reports describe excellent outcomes without neurological abnormalities, whereas others report dismal outcomes, including death (9). Though it remains variable, a good CFE outcome can be achieved with early diagnosis and supportive management.
Although the neurological disabilities associated with CFE are variable, fatal outcomes are reported often. The close monitoring of the onset of CFE in patients with a long bone fracture may allow an early diagnosis using MRI. The treatment strategy includes supportive therapy. However, considering that the occlusion of arteries by fat droplets is the pathophysiology of CFE, the prevention of the progression of thrombosis using anticoagulation therapy could become the mainstay of future treatments.
Figures and Tables
References
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996. 19:41–47.
2. Kim HJ, Lee CH, Lee SH, et al. Early development of vasogenic edema in experimental cerebral fat embolism in cats: correlation with MRI and electron microscopic findings. Invest Radiol. 2001. 36:460–469.
3. Bardana D, Rudan J, Cervenko F, Smith R. Fat embolism syndrome in a patient demonstrating only neurologic symptoms. Can J Surg. 1998. 41:398–402.
4. Finlay ME, Benson MD. Case report: magnetic resonance imaging in cerebral fat embolism. Clin Radiol. 1996. 51:445–446.
5. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999. 46:324–327.
6. Sevitt S. The significance and pathology of fat embolism. Ann Clin Res. 1977. 9:173–180.
7. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Tranpulmonary systemic fat embolism: studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994. 150:1416–1422.
8. Beers GJ, Nichols GR, Willing SJ. CT demonstration of fat embolism associated hemorrhage in the anterior commissure. AJNR Am J Neuroradiol. 1988. 9:212–213.
9. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003. 24(1):97–101.
10. Lindeque BG, Schoeman H, Dommisse G, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. J Bone Joint Surg Br. 1987. 69:128–131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download