Abstract
Purpose
The purpose of this study was to evaluate dynamic contrast-enhanced breast magnetic resonance imaging (DCE-MRI), and diffusion-weighted imaging (DWI) variables, for axillary lymph node (ALN) metastasis in the early stage of breast cancer.
Materials and Methods
January 2011-April 2015, 787 patients with early stage of breast cancer were retrospectively reviewed. Only cases of invasive ductal carcinoma, were included in the patient population. Among them, 240 patients who underwent 3.0-T DCE-MRI, including DWI with b value 0 and 800 s/mm2 were enrolled. MRI variables (adjacent vessel sign, whole-breast vascularity, initial enhancement pattern, quantitative kinetic parameters, signal enhancement ratio (SER), tumor apparent diffusion coefficient (ADC), peritumoral ADC, and peritumor-tumor ADC ratio) clinicopathologic variables (age, T stage, multifocality, extensive intraductal carcinoma component (EIC), estrogen receptor, progesterone receptor, HER-2 status, Ki-67, molecular subtype, histologic grade, and nuclear grade) were compared between patients with axillary lymph node metastasis and those with no lymph node metastasis. Multivariate regression analysis was performed, to determine independent variables associated with ALN metastasis, and the area under the receiver operating characteristic curve (AUC), for predicting ALN metastasis was analyzed, for those variables.
Results
On breast MRI, moderate or prominent ipsilateral whole-breast vascularity (moderate, odds ratio [OR] 3.45, 95% confidence interval [CI] 1.28–9.51 vs. prominent, OR = 15.59, 95% CI 2.52–96.46), SER (OR = 1.68, 95% CI 1.09–2.59), and peritumor-tumor ADC ratio (OR = 6.77, 95% CI 2.41–18.99), were independently associated with ALN metastasis. Among clinicopathologic variables, HER-2 positivity was independently associated, with ALN metastasis (OR = 23.71, 95% CI 10.50–53.54). The AUC for combining selected MRI variables and clinicopathologic variables, was higher than that of clinicopathologic variables (P < 0.05).
References
1. Park EH, Min SY, Kim Z, et al. Basic facts of breast cancer in Korea in 2014: The 10-year overall survival progress. J Breast Cancer. 2017; 20:1–11.

2. Kuijs VJ, Moossdorff M, Schipper RJ, et al. The role of MRI in axillary lymph node imaging in breast cancer patients: a systematic review. Insights Imaging. 2015; 6:203–215.

3. Qiu SQ, Zeng HC, Zhang F, et al. A nomogram to predict the probability of axillary lymph node metastasis in early breast cancer patients with positive axillary ultrasound. Sci Rep. 2016; 6:21196.

4. Roaten JB, Pearlman N, Gonzalez R, Gonzalez R, McCarter MD. Identifying risk factors for complications following sentinel lymph node biopsy for melanoma. Arch Surg. 2005; 140:85–89.

5. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011; 305:569–575.
6. Caudle AS, Hunt KK, Tucker SL, et al. American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol. 2012; 19:3144–3151.

7. Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjosne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol. 2000; 10:1464–1471.

8. Valente SA, Levine GM, Silverstein MJ, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol. 2012; 19:1825–1830.

9. Bahri S, Chen JH, Yu HJ, Kuzucan A, Nalcioglu O, Su MY. Can dynamic contrast-enhanced MRI (DCE-MRI) predict tumor recurrence and lymph node status in patients with breast cancer? Ann Oncol. 2008; 19:822–824.

10. Zaiton F, Shehata SM, Abo Warda MH, Alekrashy MA. Diagnostic value of MRI for predicting axillarylymph nodes metastasis in newly diagnosed breastcancer patients: Diffusion-weighted MRI. Egypt J Radiol Nucl Med. 2016; 47:659–667.
11. Hatakenaka M, Soeda H, Yabuuchi H, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci. 2008; 7:23–29.

12. Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol. 2007; 17:2646–2655.

13. Dietzel M, Baltzer PA, Vag T, et al. Application of breast MRI for prediction of lymph node metastases – systematic approach using 17 individual descriptors and a dedicated decision tree. Acta Radiol. 2010; 51:885–894.

14. Luo N, Su D, Jin G, et al. Apparent diffusion coefficient ratio between axillary lymph node with primary tumor to detect nodal metastasis in breast cancer patients. J Magn Reson Imaging. 2013; 38:824–828.

15. Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010; 23:619–623.

16. Dietzel M, Baltzer PA, Vag T, et al. The adjacent vessel sign on breast MRI: new data and a subgroup analysis for 1,084 histologically verified cases. Korean J Radiol. 2010; 11:178–186.

17. Knopp MV, Bourne MW, Sardanelli F, et al. Gadobenate dimeglumine-enhanced MRI of the breast: analysis of dose response and comparison with gadopentetate dimeglumine. AJR Am J Roentgenol. 2003; 181:663–676.
18. Kang DK, Kim EJ, Kim HS, Sun JS, Jung YS. Correlation of whole-breast vascularity with ipsilateral breast cancers using contrast-enhanced MDCT. AJR Am J Roentgenol. 2008; 190:496–504.

19. Esserman L, Hylton N, George T, Weidner N. Contrast-enhanced magnetic resonance imaging to assess tumor histopathology and angiogenesis in breast carcinoma. Breast J. 1999; 5:13–21.

20. Mori N, Mugikura S, Takasawa C, et al. Peritumoral apparent diffusion coefficients for prediction of lymphovascular invasion in clinically node-negative invasive breast cancer. Eur Radiol. 2016; 26:331–339.

21. Sardanelli F, Iozzelli A, Fausto A, Carriero A, Kirchin MA. Gadobenate dimeglumine-enhanced MR imaging breast vascular maps: association between invasive cancer and ipsilateral increased vascularity. Radiology. 2005; 235:791–797.

22. Wright H, Listinsky J, Quinn C, Rim A, Crowe J, Kim J. Increased ipsilateral whole breast vascularity as measured by contrast-enhanced magnetic resonance imaging in patients with breast cancer. Am J Surg. 2005; 190:576–579.

23. Han M, Kim TH, Kang DK, Kim KS, Yim H. Prognostic role of MRI enhancement features in patients with breast cancer: value of adjacent vessel sign and increased ipsilateral whole-breast vascularity. AJR Am J Roentgenol. 2012; 199:921–928.

24. Bielenberg DR, Zetter BR. The contribution of angiogenesis to the process of metastasis. Cancer J. 2015; 21:267–273.

25. Li KL, Henry RG, Wilmes LJ, et al. Kinetic assessment of breast tumors using high spatial resolution signal enhancement ratio (SER) imaging. Magn Reson Med. 2007; 58:572–581.

26. Loiselle CR, Eby PR, Peacock S, Kim JN, Lehman CD. Dynamic contrast-enhanced magnetic resonance imaging and invasive breast cancer: primary lesion kinetics correlated with axillary lymph node extracapsular extension. J Magn Reson Imaging. 2011; 33:96–101.

27. Cichon MA, Degnim AC, Visscher DW, Radisky DC. Microenvironmental influences that drive progression from benign breast disease to invasive breast cancer. J Mammary Gland Biol Neoplasia. 2010; 15:389–397.

28. Uematsu T, Kasami M, Watanabe J. Is evaluation of the presence of prepectoral edema on T2-weighted with fat-suppression 3 T breast MRI a simple and readily available noninvasive technique for estimation of prognosis in patients with breast cancer? Breast Cancer. 2014; 21:684–692.
29. Durur-Subasi I, Durur-Karakaya A, Karaman A, Seker M, Demirci E, Alper F. Is the necrosis/wall ADC ratio useful for the differentiation of benign and malignant breast lesions? Br J Radiol. 2017; 90:20160803.

Fig. 1.
Maximum-intensity-projection image (a) and ADC map (b) in a 70-year-old woman with invasive ductal carcinoma, show the method used for placing ROIs to obtain the tumor ADC, peritumor ADC. Regarding the tumor ADC, the slice with the largest tumor cross section is selected, and the largest oval ROI is placed inside the tumor (b) with reference to the MIP image (a). Mean value of the ROI 1193 × 10-6 mm2/s, is recorded as tumor ADC. For the peritumor ADC, three ROIs are placed where the ADC visually appears to be most increased on breast parenchymal tissue, adjacent to tumor contour on the ADC map (b): the three ROIs are 2328, 2062, and 2646 × 10-6 mm2/s, respectively. Maximum value 2646 × 10-6 mm2/s is recorded as peritumor ADC. ADC=apparent diffusion coefficient; ROI=region of interest
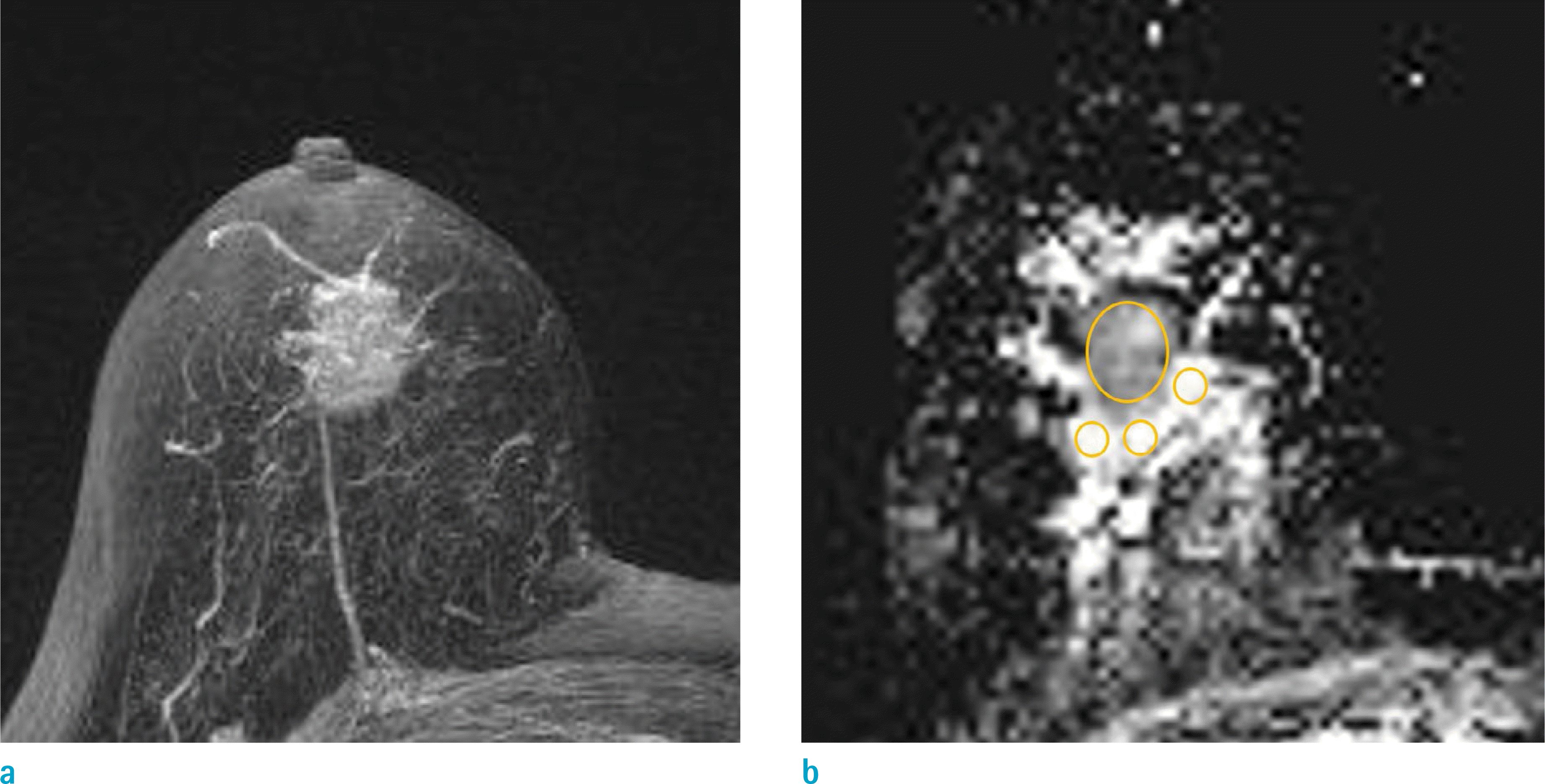
Fig. 2.
A 66-year-old woman with palpable mass (arrows) on the left breast, had pathologically confirmed invasive ductal carcinoma, with axillary lymph node metastasis after surgery. (a) Maximum-intensity-projection images show that the adjacent vessel sign is positive, and increased breast vascularity is prominent. (b) T2-weighted image shows that degree of edema around the tumor, corresponds to grade 1. Peritumor-tumor ADC ratio was calculated, using DWI (c) and ADC (d). On an ADC map, mean value of the tumor ADC, and mean value of three ROIs for peritumor ADC, were 782, 654, and 1615 × 10-6 mm2/s, respectively. So, peritumor-tumor ratio was calculated as 2.0. ADC = apparent diffusion coefficient; DWI = diffusion weighted image; ROI = region of interest
Fig. 3.
Receiver-operating characteristic (ROC) curve of predictive variables in MRI, and clinicopathologic validation, clinicopathologic validation and MRI validation. The area under the receiver operating characteristics curve (AUC) of MRI and clinicopathologic, clinicopathologic, and MRI variables, were 0.879, 0.835, and 0.729, respectively (P < 0.05).
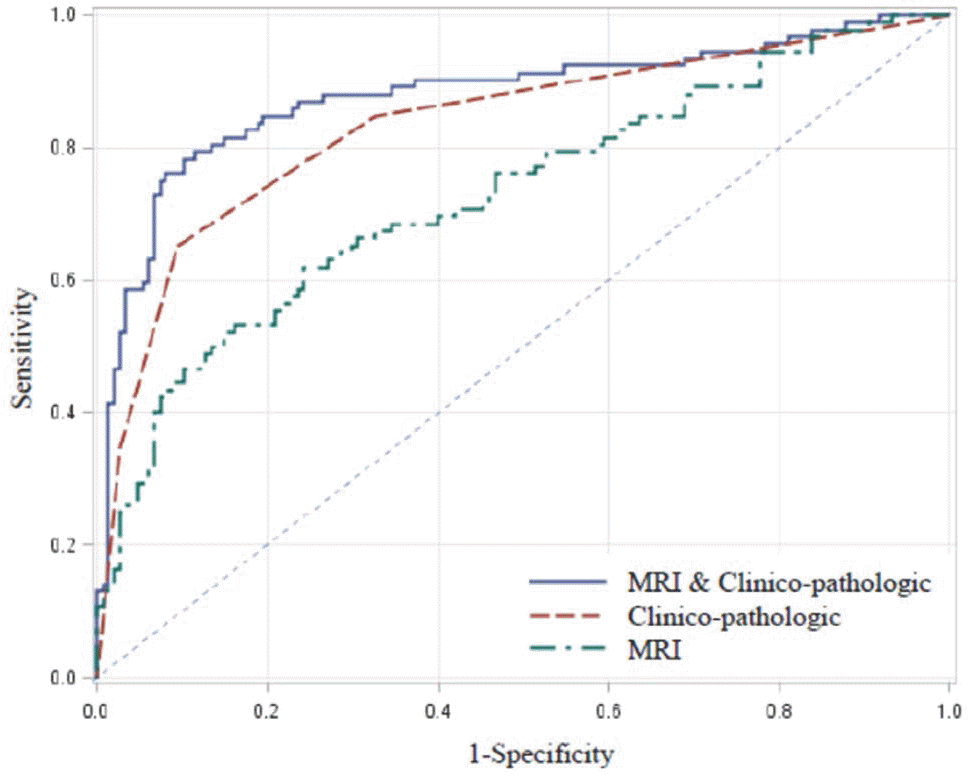
Table 1.
Comparison of Clinicopathologic Variables between No Axillary Lymph Node Metastasis and Axillary Lymph Node Metastasis
Table 2.
Comparison of DCE-MRI and DWI Variables between No Axillary Lymph Node Metastasis and Axillary Lymph Node Metastasis
| Variables | No ALN metastasis (n = 148) | ALN metastasis (n = 92) | P value |
|---|---|---|---|
| Adjacent vessel sign | < 0.001 | ||
| Negative | 87 (58.8) | 32 (34.8) | |
| Positive | 61 (41.2) | 60 (65.2) | |
| Whole breast vascularity | 0.001 | ||
| Negative | 102 (68.9) | 46 (50.0) | |
| Mild | 28 (18.9) | 20 (21.7) | |
| Moderate | 16 (10.8) | 14 (15.2) | |
| Prominent | 2 (1.4) | 12 (13.0) | |
| Quantitative parameters | |||
| E1* | 89.7 ± 65.6 | 86.6 ± 68.5 | 0.726 |
| Epeak* | 137.0 ± 104.1 | 126.4 ± 132.6 | 0.518 |
| SER* | 0.9 ± 0.5 | 1.4 ± 1.9 | 0.017 |
| TTP* | 176.3 ± 89.6 | 161.1 ± 84.1 | 0.187 |
| T2WI edema | < 0.001 | ||
| Grade 0 | 66 (44.6) | 32 (34.8) | |
| Grade 1 | 56 (37.8) | 22 (23.9) | |
| Grade 2 | 26 (17.6) | 38 (41.3) | |
| Diffusion weighted imaging | |||
| Tumor ADC* (10-6 mm2/s) | 1056 ± 249.7 | 991.1 ± 215.4 | 0.04 |
| Peritumor ADC* (10-6 mm2/s) | 1451.6 ± 331.2 | 1588.3 ± 328.5 | 0.002 |
| Peritumor-tumor ADC ratio* | 1.4 ± 0.3 | 1.6 ± 0.4 | < 0.001 |
Table 3.
Univariate Analysis for Clinicopathologic Variables Associated with Axillary Lymph Node Metastasis
Table 4.
Univariate Analysis for MRI Variables Associated with Axillary Lymph Node Metastasis
Table 5.
Multivariate Analysis for Factors Associated with Axillary Lymph Node Metastasis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download