Abstract
Purpose
To evaluate the added value of diffusion weighted imaging (DWI) to computed tomography (CT) for detecting pancreatic abnormality in patients with clinically suspected acute pancreatitis (AP).
Materials and Methods
203 patients who underwent abdomen CT and subsequent DWI to do a workup for epigastric pain were analyzed. Two blinded radiologists independently performed an interval reading based on CT image sets first, then based on combined CT and DWI image sets. The diagnostic criterion on DWI was the increased signal intensity in the pancreas to that of the spleen. For quantitative analysis, the third radiologist measured ADC value of the pancreas in each patient.
Results
For AP (n = 43), the sensitivity for detecting pancreatic abnormality increased, from 42% to 70% for reader 1 (P < 0.05) and from 44% to 72% for reader 2 (P < 0.05). For borderline pancreatitis (n = 42), the sensitivity also increased, from 10% to 26% for reader 1 (P < 0.05) and from 7% to 29% for reader 2 (P < 0.05). The mean ADC values (unit, × 10-3 mm2/s) were significantly different among the three groups (for AP, 1.09 ± 0.16; for borderline pancreatitis, 1.28 ± 0.2; for control, 1.46 ± 0.15, P < 0.05).
Acute pancreatitis (AP) is a series of acute inflammatory reactions of the pancreatic parenchyma and can range from clinically mild to lethal. In the United States, the annual incidence of AP ranges from 13 to 45/100,000 people (1). In Korea, the annual incidence of AP per 100,000 people has increased from 15.6 in 1995 to 19.4 in 2000 (2). Although AP has a 10% of median mortality, the mortality rate is increased with age and co-morbidities and reaches up to 30-50% particularly in necrotizing pancreatitis (12345). AP is clinically defined by at least the first two of the following three features: (a) clinically suggesting findings of AP (epigastric pain often radiating to the back), (b) elevated serum amylase or lipase levels more than three folds than their normal range, (c) characteristic findings on computed tomography (CT), magnetic resonance imaging (MRI) or trans-abdominal ultrasonography (6). If AP is not diagnosed on the basis of the first two criteria, then imaging evaluation may be necessary for diagnosing AP and determining subsequent patient care (6).
Contrast-enhanced CT is the primary imaging tool for evaluating the pancreas because it is generally and widely available for these acutely ill patients and has a sufficient degree of accuracy (7). In the evaluation of AP by CT, the sensitivity is 77-92% and specificity is almost 100% (8). However, despite clinical suspicion of AP, 14-28% of patients can show normal appearance in the pancreas on contrast-enhanced CT in the emergency room (ER) setting (89). Moreover, the mortality rate of patients with AP can be increased when prompt appropriate treatment is delayed (5). Therefore, radiological diagnosis of AP is of great importance, particularly for the borderline pancreatitis group who does not meet the first two diagnostic criteria because they can benefit from prompt treatment without radiological diagnostic delay. In such cases, currently, MRI can be a promising alternative imaging modality (1011).
Diffusion weighted imaging (DWI) is an emerging functional MR technique that derives its signals from differences in the movement of water protons (12). DWI has a variety of clinical advantages such as rapid image acquisition, and no contrast media administration. Therefore, it can be easily incorporated into ER examinations.
Several investigators have reported promising results in characterizing pathologic conditions in the pancreas by using quantitative apparent diffusion coefficient (ADC) measurement (131415). However, to the best of our knowledge, few studies have been conducted to evaluate the added value of DWI in determining AP since Shinya et al. (16) reported a case in which DWI showed a high signal intensity (SI) in the setting of AP. Therefore, the purpose of our study was to evaluate the added value of DWI to CT for detecting pancreatic abnormality in patients with clinically suspected AP.
This prospectively collected and retrospectively evaluated study was approved by the Institutional Review Board, and informed consent from patients was received.
From December 2012 to January 2014, a total of 214 patients who visited ER with acute onset of abdominal pain and met the following inclusion criteria were initially eligible. The inclusion criteria were as follows: (i) abdominal pain was defined as the acute onset of a persistent and severe epigastric pain that was often radiating to the back, which was based on the previously established criteria (6), (ii) patients having documentation of serum amylase or lipase levels, (iii) patients underwent abdomen-pelvis CT within three days from the onset of symptoms and (iv) subsequent magnetic resonance cholangiopancreatography (MRCP) was performed within four days from the CT exams. Among them, 11 patients who had a poor image quality of MRCP examination to obtain ADC value of pancreas were excluded. Eighty five patients (41 men, 44 women; mean age, 61.3 years; age range, 25-85 years) had serum amylase or lipase beyond the normal range. Among them, 43 patients (18 men, 25 women; mean age 61.6 years; age range, 25-85 years), whose serum amylase or lipase levels exceeded three times of the normal range, were finally enrolled as AP group. Of the rest 42 patients (23 men, 19 women; mean age, 61.1 years; age range, 25-83 years), they were defined as borderline AP group as having increased levels of serum amylase or lipase, but which were less than threefold of the upper limit of normal, as well as typical symptoms. A total of 118 patients (53 men, 65 women; mean age, 64.9 years; age range, 28-88 years) whose laboratory examinations revealed the normal range of serum amylase and lipase were finally categorized as control group. The normal cut-off level of serum amylase was 168 U/L, and that of serum lipase was 60 U/L (6). The case accrual process is summarized in Figure 1.
A 128-detector row CT scanner (Definition AS+, Siemens Healthcare, Forchheim, Germany) was used to perform the abdomen-pelvis CT scan. All patients were in the supine position and were scanned from the lung base to the pubic symphysis. We performed a portal venous phase scan. CT scanning was performed using a fixed time delay method of 70 seconds after intravenous administration of the contrast agent. Iobitridol (Xenetix 300®; Guerbet, Roissy, France) was used as a contrast agent, and 2 mL per kilogram of body weight was injected intravenously at a rate of 3 mL/second through the antecubital vein. The scanning parameters were as follows: tube voltage, 120 kVp; collimation, 128 × 0.6 mm; rotation speed, 0.5 second; pitch, 0.8; reconstruction thickness, 5 mm; and no reconstruction overlap. Automatic exposure control (caredose 4D, Siemens Healthcare) was activated to decrease the radiation dose, however, automatic tube potential modulation (careKV, Siemens Healthcare) was not switched on. Sagittal and coronal reformatted images were generated with a thickness of 3 mm.
A 3.0 tesla (T) MR scanner (Achieva-TX; Philips Medical Systems, Best, The Netherlands) was used for MRCP examinations. Routine MRI protocols consisted of an axial and coronal single-shot fast spin echo (FSE), an axial T1-weighted fat-saturated spoiled gradient echo (THRIVE) sequence and respiratory-triggered three-dimensional (3D) T2 weighted FSE. Axial DWI was routinely performed as part of MRCP. The same parameters, such as slice thickness (5 mm), gap (1 mm) and field of view (FOV, 350 × 350 mm), were used to acquire respiratory-triggered DWI and to match the pancreas on the axial T2WI. Spin echo type echo planar imaging technique was used for DWI. Two b factors of 0, 800 s/mm2 were used. The number of excitations was 3. The detailed parameters of each sequence are summarized in Table 1.
Two radiologists with seven years of experience in reading MRCP independently performed a four-week-interval reading. Both radiologists were blinded to all clinical information, including serum amylase, lipase and patient's medical history. At the first reading session, they independently reviewed only CT imaging data from all patients. The diagnostic criteria for AP on CT include swelling of the pancreas, peripancreatic fat infiltration, peripancreatic fluid collection or parenchymal necrosis (6). Each reader scored the probability of AP with a five-point confidence scoring system, and the scoring system was as follows: 1, Definitely normal; 2, Probably normal; 3, Possibly AP; 4, Probably AP; and 5, Definitely AP. At the second reading session, each reader scored their confidence level for AP with the combined image set of DWI (b values of 800 mm2/s) and CT images using the same scoring system. On DWI, AP was defined as locally or diffusely increased SI to that of spleen. This definition was based on the literature which included qualitative and quantitative observations (14161718192021). In the general population, the normal ADC value of the pancreas is within a range of 1.2-1.9 × 10-3 mm2/s and that of the spleen is 0.9-1.6 × 10-3 mm2/s (20). In contrast, in patients with AP, it is known that the ADC value of the pancreas could be decreased to 0.9-1.5 × 10-3 mm2/s, which indicates that the pancreas can show an increased SI to that of the spleen on DWI in AP (1417). Ma et al. (21) also observed that the signal intensity ratios (SIR) of pancreas and liver on DWI were significantly higher in patients with AP (SIR = 2.63) than in the control group (SIR = 1.64). Based on these observations, we defined AP on DWI as having increased SI to that of the spleen. In case of concordant decisions on both DWI and CT, the final decision was made accordingly. When the decisions on DWI were different from those on CT, the readers rated final score according to DWI rather than CT.
To obtain ADC value of pancreas parenchyma, all measurements were performed by the third radiologist with three years of experience in measuring ADC values in the pancreas. The radiologist was also blinded to clinical and laboratory data. Pancreas was divided into head, body and tail. After referencing DWI, the radiologist measured the pancreas ADC value on the ADC map by placing a circular region of interest (average area: 16.62 mm2) in each pancreatic segment. A representative ADC value in each patient was obtained by averaging the measured ADC values. To obtain ADC ratio (pancreas/spleen) in each patient, additional measurements were performed in the spleen. To obtain a representative ADC value of the spleen in each patient, two circular ROIs were randomly placed in the spleen, and the measured ADC values were averaged.
Sensitivity was calculated on the assumption that a confidence level of 3 or higher was positive for the diagnosis of AP. Under this assumption, the McNemar test was used to evaluate the added value of DWI for detecting AP. Analysis of variance was used to compare mean ADC values and mean ADC ratios (pancreas/spleen) among the three groups. Receiver operating characteristic (ROC) curve analysis was used to calculate area under the curve (AUC) and to determine the cut-off value of ADC to diagnose AP. A pairwise comparison of ROC curves was used to compare AUCs between the ADC and ADC ratio (pancreas/spleen). All statistical analyses were performed by using statistical software (MedCalc for Windows, version 12.7.1.0; MedCalc Software, Mariakerke, Belgium). A P value less than 0.05 was considered to indicate a significant difference.
Of the 85 patients with pancreatitis, 81 patients underwent abdomino-pelvic CT scan within 1 day (range, 1-3 days) after the presentation of symptoms. Subsequent MRCP was performed within 2.4 days (range, 1-3 days) in patients with AP and within an average of 3.5 days (range, 1-4 days) in patients with borderline pancreatitis. Among those patients with AP, gallstone (n = 20) was the most common cause of AP, followed by pancreatic neoplasms (n = 6), alcohol drinking (n = 1), common bile duct stricture (n = 1) and other unknown causes (n = 15). The means and standard deviations (SDs) of serum amylase and lipase levels of patients with AP were 1728 ± 1630 U/L and 2861 ± 2602 U/L, respectively, and those of patients with borderline pancreatitis were 123 ± 44 U/L and 92 ± 31 U/L, respectively. Of the 118 patients with control group, those were 86 ± 69 U/L and 35 ± 13 U/L, respectively.
For patients with AP, after the additional reading of DWI, 12 additional patients were assessed by both readers as having abnormal increased SI in the pancreas. The sensitivity for detecting radiological pancreas abnormality increased, from 42% to 70% for reader 1 (P < 0.05) and from 44% to 72% for reader 2 (P < 0.05) after combined reading of DWI and CT (Fig. 2).
For patients with borderline pancreatitis, after the additional reading of DWI, seven and nine additional patients were assessed by readers 1 and 2, respectively, as having abnormal increased SI in the pancreas. The sensitivity for detecting radiological pancreas abnormal findings also increased, from 10% to 26% for reader 1 (P < 0.05) and from 7% to 29% for reader 2 (P < 0.05) (Fig. 3). The cross tables of the interpretation results for both readers on CT and additional DWI are summarized in Tables 2 and 3.
For control group, all patients showed no remarkable abnormal findings in the pancreas on both CT and DWI.
The mean pancreatic ADC values (unit, ×10-3 mm2/s) were significantly different among the three groups (for AP, 1.09 ± 0.16; for borderline pancreatitis, 1.28 ± 0.2; for control, 1.46 ± 0.15; P < 0.05). Figure 4 demonstrates the box and whisker plot that was used to compare mean pancreatic ADC values of the three groups. The AUC was 0.964 (95% confidence interval [CI]: 0.923-0.987). When an ADC value of 1.29 × 10-3 mm2/s was used as a cut-off value for distinguishing AP from control group, a maximum accuracy of 90% was estimated with a sensitivity of 91% and a specificity of 90%.
The mean ADC ratios (pancreas/spleen) were also significantly different among the three groups (for AP, 1.35 ± 0.22; for borderline pancreatitis, 1.56 ± 0.28; for control, 1.81 ± 0.22; P < 0.05). The AUC was 0.923 (95% CI: 0.871-0.959). When an ADC ratio of 1.55 was used as a cut-off value, a maximum accuracy of 88% was estimated with a sensitivity of 86% and a specificity of 89%. There was no significant difference between the AUCs of the pancreatic ADC value and ADC ratio (P > 0.05) (Fig. 5).
Our results showed that sensitivity for detecting pancreatic abnormality significantly increased by approximately 30% after adding DWI to CT in the AP group who visited the ER. In addition, in cases of borderline pancreatitis, the sensitivity also increased by approximately 20% after the additional reading of DWI. As CT can demonstrate normal finding in the pancreas in 14-28% out of patients with AP (89), if the CT findings were negative in the pancreas in patients having clinical suspicion of AP, DWI may be an adjunct to CT for detecting pancreatic abnormality. Therefore, we suggest that supplementary DWI examinations may be helpful in detecting pancreatic abnormality when the findings on CT are negative in patients clinically suspected of having AP in the ER. According to the most recent diagnostic criteria on AP (6), imaging can play an important role in the diagnosis of AP, particularly for patients having borderline pancreatitis, which was defined as acute epigastric pain, as well as increased serum amylase or lipase levels, which did not increase over three times of the normal cutoff values. Therefore, we believe that those patients may benefit from additional DWI because prompt treatment can be made without a delay in diagnosis.
Our results correspond well with those of previous studies (1622). Shinya et al. (16) reported a case in which DWI showed a high SI in the setting of AP. Lee et al. (22) reported that DWI can be useful for detecting gallstone pancreatitis more clearly than non-enhanced CT when iodine contrast is not available. They also presented that AP and infected peripancreatic fluid collection showed diffusion restriction. On the other hand, our results are in contrast to that of a previous study, which concluded that there were no significant differences between using CT and DWI to evaluate AP (19). A discrepancy between our study and theirs could be because their study population was relatively small (n = 11) and as such, did not have the power to evaluate differences between CT and DWI. Moreover, most CT images showed diffuse pancreatic swelling and mild fluid collection around the pancreas, which is compatible with AP at the time of diagnosis. Thus, we believe that it might be difficult to properly evaluate significant differences between CT and DWI under those clinical settings.
The established mechanism of diffusion restriction in tumors is known to be due to increased tissue cellularity, viscosity and the integrity of cell membranes (12). Like in tumors, DWI also has the capability of detecting inflammatory processes in the abdomen (2324). Oto et al. (23) reported that in patients with Crohn's disease, bowel segments with active inflammation revealed diffusion restriction on DWI. Currently, it is not fully understood why diffusion restriction occurs in inflammatory lesions. In our opinion, diffusion restriction might occur when the interstitial space is narrowed due to the aggregation of inflammatory cells and inflammatory cellular edema (25).
Our study revealed that AP group demonstrated lower ADC value and ADC ratio than those of patients with borderline pancreatitis and control group. Our results correspond well with several previous studies (1315). Kamisawa et al. (13) reported that ADC values were significantly lower in autoimmune pancreatitis (1.012 ± 0.112 × 10-3 mm2/s) than in pancreatic cancer (1.249 ± 0.113 × 10-3 mm2/s) and normal pancreas (1.491 ± 0.162 × 10-3 mm2/s) (P < 0.001). Thus, they concluded that ADC was useful in discriminating autoimmune pancreatitis from pancreatic cancer and normal pancreas. Hocaoglu et al. (15) also reported that there was a significant reduction in mean pancreatic ADC among the AP (1.46 ± 2.80 × 10-3 mm2/s) relative to the healthy subjects (1.69 ± 2.26 × 10-3 mm2/s).
In our study, we performed DWI as part of scheduled MRCP. Although gallstone is one of the common causes of AP (26), CT often does not reliably demonstrate gallstones (27), whereas MRCP is excellent for the evaluation of stones in the biliary tree, with a high sensitivity and accuracy of 88-95% and 89-96%, respectively (27). DWI can be easily incorporated into the MRCP protocol and can be rapidly obtained. Moreover, DWI needs no contrast media, thus can be safely performed for the patients with renal dysfunction. Therefore, we suggest that complementary DWI could be part of scheduled MRCP to detect abnormal increased SI in the pancreas in cases of clinically suspected AP.
There were several limitations to our study. First, most patients underwent CT within one day after onset of symptoms. It may lead to the false negative of CT for diagnosing AP. In addition, the time interval between CT and DWI in AP and borderline AP group may lead to evolution of pancreatitis with development of pathologic abnormalities, which may then manifest in imaging. Therefore, the second imaging modality performed later might have higher chances to detect an abnormality in clinically evolving pancreatitis. Second, we did not determine whether DWI combined with routine MRCP could improve the sensitivity and accuracy in the diagnosis of AP. Although Ma et al. (21) reported that additional DWI to routine MRI could increase the sensitivity and accuracy in diagnosing AP, our study purpose was focused on comparing CT with DWI.
In conclusion, the sensitivity for detecting pancreatic abnormality significantly increased after adding DWI to CT in patients with clinically suspected AP.
Figures and Tables
Fig. 2
Added value of additional DWI for identifying radiological abnormality in the pancreas in patients with acute pancreatitis (AP), as demonstrated in a 61-year-old woman. She visited the emergency room with acute epigastric pain, and her laboratory test revealed elevated serum amylase and lipase levels (2586 U/L and 2416 U/L, respectively), which were over three times of the normal limits. The normal cut-off level of serum amylase is 168 U/L, and that of serum lipase is 60 U/L. (a) Axial CT image on portal venous phase showing no significant abnormality in the pancreas. At the first reading session, both readers recorded that there was no remarkable abnormality in the pancreas on this axial CT image. (b) Axial diffusion weighted image (b = 800 s/mm2) showing diffusely increased signal intensity throughout the pancreas, which is higher than that of spleen. After the combined reading of DWI and CT on second reading session, both readers changed their confidence score from 1 to 5 (definite AP). (c) Axial diffusion weighted image (b = 800 s/mm2) of the pancreas in a 60-year-old woman out of control group.
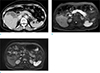
Fig. 3
Added value of additional DWI for detecting radiological pancreatic abnormality in patients with borderline pancreatitis, as demonstrated in a 71-year-old man. He visited the emergency room with acute epigastric pain and obstructive jaundice, and his initial laboratory test revealed an elevated serum lipase (99 U/L) level, which was less than three folds of normal serum lipase, and a normal level of serum amylase (129 U/L). The normal cut-off level of serum amylase is 168 U/L, and that of serum lipase is 60 U/L. (a) There were no significant abnormal findings in the pancreas on the portal venous phase of axial CT images. Both readers reported that there was no definite evidence of acute pancreatitis (AP) (confidence score of 1) at the first reading session. (b) On axial diffusion weighted image (b = 800 s/mm2), the pancreas showed a diffusely increased signal intensity throughout the whole pancreas parenchyma, which was similar to that of the spleen. Both readers changed their confidence score from 1 to 5 (definite AP) after the combined reading of DWI and CT on second reading session.
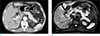
Fig. 4
Box and whisker plot comparing the mean ADC values of pancreas among acute pancreatitis (AP), borderline pancreatitis, and control groups. The mean ADC values (unit, × 10-3 mm2/s) were significantly different among the three groups (for AP, 1.09 ± 0.16; for borderline pancreatitis, 1.28 ± 0.2; for control, 1.46 ± 0.15, P < 0.0001). The middle line in each box represents the median. The lower and upper boundaries of the boxes represent the lower and upper quartiles (25th and 75th percentiles, respectively). The whiskers indicate the range from the maximum to the minimum calculated ADC values. ▼△ = outlier

Fig. 5
Receiver operating characteristic curves for distinguishing acute pancreatitis from control group for pancreatic ADC value (solid line) and ADC ratio (pancreas/spleen, dashed line). The area under the curve (AUC) of the pancreatic ADC value was not significantly different from that of the ADC ratio (AUC, 0.964; 0.923, respectively, P = 0.0514).

Table 1
Magnetic Resonance Cholangiopancreatography Sequence Parameters

Table 2
Pancreas Abnormality on CT and Combined Diffusion Weighted Imaging and CT in Acute Pancreatitis Group for Both Readers

| CT (−) | CT (+) | Total | |
|---|---|---|---|
| Reader 1 | |||
| DWI/CT (−) | 13 | 0 | 13 |
| DWI/CT (+) | 12 | 18 | 30 |
| Total | 25 | 18 | 43 |
| Reader 2 | |||
| DWI/CT (−) | 12 | 0 | 12 |
| DWI/CT (+) | 12 | 19 | 31 |
| Total | 24 | 19 | 43 |
References
1. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013; 144:1252–1261.
2. Kim CD. Current status of acute pancreatitis in Korea. Korean J Gastroenterol. 2003; 42:1–11.
3. Arvanitakis M, Koustiani G, Gantzarou A, et al. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging-a comparative study. Dig Liver Dis. 2007; 39:473–482.
4. Lankisch PG, Assmus C, Lehnick D, Maisonneuve P, Lowenfels AB. Acute pancreatitis: does gender matter? Dig Dis Sci. 2001; 46:2470–2474.
5. de Beaux AC, Palmer KR, Carter DC. Factors influencing morbidity and mortality in acute pancreatitis; an analysis of 279 cases. Gut. 1995; 37:121–126.
6. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111.
7. Hill MC, Barkin J, Isikoff MB, Silverstein W, Kalser M. Acute pancreatitis: clinical vs. CT findings. AJR Am J Roentgenol. 1982; 139:263–269.
8. Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985; 156:767–772.
9. Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994; 193:297–306.
10. Viremouneix L, Monneuse O, Gautier G, et al. Prospective evaluation of nonenhanced MR imaging in acute pancreatitis. J Magn Reson Imaging. 2007; 26:331–338.
11. Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999; 211:727–735.
12. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007; 188:1622–1635.
13. Kamisawa T, Takuma K, Anjiki H, et al. Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. Am J Gastroenterol. 2010; 105:1870–1875.
14. Yencilek E, Telli S, Tekesin K, et al. The efficacy of diffusion weighted imaging for detection of acute pancreatitis and comparison of subgroups according to Balthazar classification. Turk J Gastroenterol. 2014; 25:553–557.
15. Hocaoglu E, Aksoy S, Akarsu C, Kones O, Inci E, Alis H. Evaluation of diffusion-weighted MR imaging in the diagnosis of mild acute pancreatitis. Clin Imaging. 2015; 39:463–467.
16. Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. Acute pancreatitis successfully diagnosed by diffusion-weighted imaging: a case report. World J Gastroenterol. 2008; 14:5478–5480.
17. Thomas S, Kayhan A, Lakadamyali H, Oto A. Diffusion MRI of acute pancreatitis and comparison with normal individuals using ADC values. Emerg Radiol. 2012; 19:5–9.
18. Yoshikawa T, Kawamitsu H, Mitchell DG, et al. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006; 187:1521–1530.
19. Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The efficacy of diffusion-weighted imaging for the detection and evaluation of acute pancreatitis. Hepatogastroenterology. 2009; 56:1407–1410.
20. Kilickesmez O, Yirik G, Bayramoglu S, Cimilli T, Aydin S. Non-breath-hold high b-value diffusion-weighted MRI with parallel imaging technique: apparent diffusion coefficient determination in normal abdominal organs. Diagn Interv Radiol. 2008; 14:83–87.
21. Ma J, Xia L, Ma X, Zhu L, Zhang G, Yang Y. Diagnostic value of diffusion weighted imaging in acute pancreatitis. Modern Diagnosis & Treatment. 2011; 4:193–196.
22. Lee NK, Kim S, Kim GH, et al. Diffusion-weighted imaging of biliopancreatic disorders: correlation with conventional magnetic resonance imaging. World J Gastroenterol. 2012; 18:4102–4117.
23. Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn's disease. Acad Radiol. 2009; 16:597–603.
24. Ream JM, Dillman JR, Adler J, et al. MRI diffusion-weighted imaging (DWI) in pediatric small bowel Crohn disease: correlation with MRI findings of active bowel wall inflammation. Pediatr Radiol. 2013; 43:1077–1085.
25. Tsuchiya K, Katase S, Yoshino A, Hachiya J. Diffusion-weighted MR imaging of encephalitis. AJR Am J Roentgenol. 1999; 173:1097–1099.
26. Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006; 33:323–330.
27. Bortoff GA, Chen MY, Ott DJ, Wolfman NT, Routh WD. Gallbladder stones: imaging and intervention. Radiographics. 2000; 20:751–766.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download