Abstract
Background and Purpose
Subjective cognitive decline has been proposed as a potential indicator of the preclinical state of Alzheimer's disease (AD). The results of the studies of cortical atrophy on brain MRIs in subjects with subjective cognitive decline are inconsistent across the literatures. We investigated whether subjects with subjective cognitive decline had less gray matter volume compared to controls without subjective cognitive decline as per brain MRI.
Methods
Thirty-six subjects with subjective cognitive decline and thirty-three controls without subjective cognitive decline were recruited retrospectively from among the patients who had visited the department of neurology at Inha University Hospital between January 2008 and December 2010. All subjects had undergone a brain MRI scan including 3D T1-weighted spoiled gradient recalled echo imaging. We used voxel-based morphometry (VBM) to examine gray matter volumes between the two groups, after controlling for age, sex, education, and total intracranial volumes (TIV).
Results
There were no significant differences in age, gender, education, and TIV between the two groups. In comparison to controls without subjective cognitive decline, subjects with subjective cognitive decline showed gray matter atrophy in the left superior and medial frontal gyri, left superior and inferior parietal lobules, and right precuneus and insular in the VBM analysis.
Persons with subjective cognitive decline are reported to experience a decline in cognitive capacity without measurable cognitive deficits on objective testing.1 In a meta-analysis, subjective cognitive decline was present in about 17% of healthy elderly.2 The pathophysiological process of Alzheimer's disease (AD) is thought to begin many years before the diagnosis of AD dementia.3 Subjective cognitive decline has been proposed as a potential indicator of the preclinical state of AD.34 Older people with subjective cognitive decline are twice as likely to develop dementia as individuals without subjective cognitive decline.5 In a meta-analysis, approximately 2.3% and 6.6% of older people with subjective cognitive decline progressed to dementia and mild cognitive impairment (MCI) per year.5
The existence of subjective cognitive decline does not ascertain progression to clinical AD. Long-term studies, with a follow-up period of over 4 years, showed that 14% of individuals with subjective cognitive decline develop dementia and about 27% go on to develop MCI.1 Subjective cognitive decline may be non-specific and may result from other causes including attention difficulties, depression, sleep disorders, and drug side-effects.16 Therefore, detection of sensitive markers of preclinical AD in individuals with subjective cognitive decline is critical before the introduction of a potential disease-modifying treatment. The underlying biological changes as assessed by biomarkers have been examined in subjects with subjective cognitive decline. A study on subjects with subjective cognitive decline found that cerebrospinal fluid (CSF) 42-mer amyloid β-protein (Aβ42) alone is a superior biomarker over total tau, hyperphosphorylated tau, or a combination of all three biomarkers in predicting progression to MCI or AD.7 Previous studies also demonstrated brain atrophy through magnetic resonance imaging (MRI),8910 and increased hypometabolism, as revealed through positron emission tomography (PET), in characteristic brain regions affected by AD.11 MRI-based methods have the advantage of being non-invasive and relatively cost-effective compared to PET neuroimaging and CSF analysis. Brain MRIs are also performed routinely for evaluating the cause of cognitive decline.
Studies have reported volume reductions of the entorhinal cortex and the hippocampus in subjects with subjective cognitive decline compared to healthy controls without subjective cognitive decline.18 However, the results of the studies of cortical atrophy in subjects with subjective cognitive decline are inconsistent across the literatures.910 In a recent study, subjects with subjective cognitive decline showed gray matter atrophy in the left frontal gyrus, right calcarine gyrus, precuneus, lingual gyrus, temporal gyrus, and cingulate areas, compared to normal controls without subjective cognitive decline.9 In another study, measures of cortical thickness showed no significant difference between subjects with subjective cognitive decline and cognitively healthy controls without subjective cognitive decline.10 We investigated whether subjects with subjective cognitive decline had less gray matter volume compared to healthy controls without subjective cognitive decline as per brain MRI.
Thirty-six subjects with subjective cognitive decline and thirty-three healthy controls without subjective cognitive decline were recruited retrospectively from among the patients who had visited the department of neurology at Inha University Hospital between January 2008 and December 2010. The subjects with subjective cognitive decline were 50 years or older and were diagnosed by the criteria of the Subjective Cognitive Decline Initiative.4 They complained of self-experienced persistent decline in cognitive capacity in comparison with a previously normal status and had scores more than 1.0 standard deviation below age and education-adjusted normative means on all subtests of the Seoul Neuropsychological Screening Battery-Dementia version.12 They had undergone a brain MRI scan including 3D T1-weighted spoiled gradient recalled echo (SPGR) imaging. The normal control subjects without subjective cognitive decline were selected from among the patients who had visited the neurology clinic because of chronic headache or dizziness and had undergone a brain MRI scan including 3D T1-SPGR imaging between January 2008 and December 2010. The control subjects were at least 50-years-old and did not complain of self-experienced persistent decline in cognitive capacity in comparison with a previously normal status and any impairment in activities of daily living. They also did not have any of 28 diseases or a history suggestive of a decrease in cognitive function.13 They included the following: stroke or transient ischemic attack, seizures, Parkinson's disease, multiple sclerosis, cerebral palsy, Huntington's disease, encephalitis, meningitis, brain surgery, surgery to clear arteries to the brain, diabetes that required insulin for control, hypertension that was not well controlled, cancer other than skin cancer diagnosed within the past three years, shortness of breath while sitting still, use of home oxygen, heart attack with changes in memory, walking, or solving problems lasting at least 24 hours, kidney dialysis, liver disease, hospitalization for mental or emotional problems in the past five years, current use of medications for mental or emotional problems, alcohol consumption greater than three drinks each day, abuse of drugs in the past five years, treatment for alcohol abuse in the past five years, unconsciousness for more than one hour other than during surgery, overnight hospitalization because of a head injury, illness causing a permanent decrease in memory or other mental functions, trouble with vision that impairs reading ordinary print even with glasses on, and difficulty understanding conversations because of hearing problems even if wearing a hearing aid. They were matched randomly for age, gender, and education with subjects with subjective cognitive decline.
Patients with the following diseases or conditions were excluded: severe or unstable systemic diseases; abnormal laboratory tests such as vitamin B12 or folate deficiency, positive syphilis serology, and uncontrolled thyroid disease; a major neurological disease such as Parkinson's disease, normal pressure hydrocephalus, seizure, and stroke; alcoholism or drug abuse; the Korean version of Geriatric Depression Scale score above 18,14 major depression, or psychiatric diseases; and severe white matter hyperintensities (Fazekas scale 3),15 any lacunes, cerebral hemorrhages, cortical stroke, or brain tumors on brain MRI. This study was approved by the Institutional Review Board of Inha University Hospital.
SPGR imaging was performed using a 1.5-T GE Signa MRI scanner (signal HDX, General Electric, Milwaukee, WI, USA) with the following imaging parameters: repetition time=11.2 ms, echo time=4.9 ms, field of view=240×240 mm, slice thickness=1.2 mm, no interslice gap, number of excitation=1, and acquisition matrix=250×160.
All images were saved in Digital Imaging and Communications in Medicine format and changed to an appropriate format for voxel based morphometry (VBM) using MRIcro. VBMs were performed using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). We used the standard template that was provided by the Montreal Neurological Institute to perform spatial normalization with non-linear transformation. The purpose of performing this step was to correct for global differences in brain shape. Normalized images for each subject were segmented into gray and white matter and CSF using the default SPM brain tissue prior probability maps. After normalization and segmentation, smoothing was performed with a 12-µL full-width at half-maximum Gaussian kernel for preprocessing. After image processing, voxel-based comparisons between the two groups were made using two-sample t-tests. Age, gender, educational levels, and total intracranial volume (TIV), obtained automatically using SPM5, were used as covariates. Only the regions that differed in more than 100 voxels between the two groups were analyzed. Areas with voxel levels of p<0.005, uncorrected, were regarded as significant. The x, y, z coordinates of the areas of significant correlation obtained from the analyses were first converted into Talairach coordinates and then the specific anatomy region and the Brodmann area were identified using the Talairach Daemon Client.
Demographic and clinical characteristics were compared using student t-tests for continuous variables and a chi-square test for categorical variables. The effect of age and subjective cognitive decline on the gray matter volumes was tested by multiple linear regression method. Significance for all tests was set at α=0.05, two-tailed. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
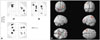
There were no significant differences in age, gender, education, TIV, and total gray matter volumes between subjects with subjective cognitive decline and controls without subjective cognitive decline (Table 1). The neuropsychological findings in subjects with subjective cognitive decline are presented in Table 2. The distribution of total gray matter volumes according to age in both groups is shown in Fig. 1. The total gray matter volumes were not significantly associated with age (p=0.49) and the presence of subjective cognitive decline (p=0.78). However, in comparison to controls without subjective cognitive decline, subjects with subjective cognitive decline showed gray matter atrophy in the left superior and medial frontal gyri, left superior and inferior parietal lobules, and right precuneus and insular in the VBM analysis (Table 3, Fig. 2).
We discovered significant macrostructural gray matter loss in the brains of subjects with subjective cognitive decline compared to normal controls without subjective cognitive decline. This suggests that subjects with subjective cognitive decline are neuroanatomically different from controls without subjective cognitive decline. Of special note, there was significant gray matter atrophy of the right precuneus and insula, left superior and medial frontal gyri, and left superior and inferior parietal lobules in subjects with subjective cognitive decline compared to controls without subjective cognitive decline. In another study, subjects with subjective cognitive decline showed gray matter atrophy in the left frontal gyrus and right precuneus compared to normal controls, which is similar to the results of this study.9 In a recent study, individuals with subjective cognitive decline showed greater similarity to an AD gray matter atrophy pattern compared to control subjects without subjective cognitive decline.16
In a previous study, the regions which were vulnerable to early deposition of amyloid β protein included the precuneus, posterior cingulate, lateral parietal region, and medial and lateral prefrontal regions.17 The areas were also compatible with the regions within the default mode network. The impaired default mode network has been reported in AD and longitudinal data showed a correlation with clinical symptoms worsening.18 Non-demented adults carrying a familial AD gene mutation also showed decreased connectivity in the default mode network.19 The brain regions showing gray matter atrophy in subjects with subjective cognitive decline in this study are compatible with the areas vulnerable to early amyloid deposition. This suggests that subjects with subjective cognitive decline may be in the preclinical stage of AD. Subjects with subjective cognitive decline in this study may be in stage 2 of preclinical AD with amyloid deposition and early neurodegeneration.3
Atrophy of the insular cortex observed in the subjects with subjective cognitive decline is an interesting finding of this study. Many studies have reported that the insular cortex is involved in a variety of processing events, including interoceptive awareness, multimodal sensory integration, emotional processing, and regulation.2021 The insular cortex is associated with neuropsychiatric symptoms, changes in cardiovascular and autonomic control, and mortality in AD.22 A recent study showed that atrophy of the insular cortex was detected in patients with mild AD.23 The insular cortex is connected to the hippocampus and entorhinal cortex.23 Atrophy of the insular cortex in subjective cognitive decline may indirectly reflect neuronal degeneration in the hippocampus and entorhinal cortex.
There are some limitations to our study. The first limitation regarding the study design was that this was not a prospective study. Therefore, we could not obtain any information on risk factors such as the presence of apolipoprotein E ε4 and a family history of dementia. Second, the control subjects without subjective cognitive decline were not evaluated with extensive neuropsychological tests. Some control subjects with MCI might have been included. Third, there is a possibility that we may not have obtained more significant findings because of the small sample size of this study. Fourth, biomarkers that reflect the presence of cerebral Aβ deposits were not evaluated in the subjects. The causes of subjective cognitive decline are heterogeneous. We may not have obtained more significant findings because some subjects without cerebral Aβ deposits might have been included in the subjective cognitive decline group. In future, we need to investigate brain atrophy patterns in individuals with subjective cognitive decline, in whom the presence of cerebral Aβ deposits is confirmed with a biomarker.
Despite these limitations, our study has some strengths; we observed that persons with subjective cognitive decline encountered in clinical settings have greater similarity to an AD gray matter atrophy pattern compared to cognitively normal individuals without subjective cognitive decline. Therefore, clinicians should follow-up patients with subjective cognitive decline longitudinally and administer pharmacological and non-pharmacological treatments whenever necessary.
Figures and Tables
Fig. 1
The distribution of total gray matter volumes according to the age in subjects with subjective cognitive decline (black dots, solid line) and those without subjective cognitive decline (gray dots, broken line). The total gray matter volumes were not significantly associated with age and the presence of subjective cognitive decline.

Fig. 2
Statistical parametric maps (SPM) (A) and three dimensional rendering (B) of gray matter atrophy in subjects with subjective cognitive decline compared to controls without subjective cognitive decline. Meaningfully reduced gray matter distributions were located in the right precuneus and insular, left superior and inferior parietal lobules, and left superior and medial frontal gyri. Results were considered statistically significant at p<0.005, cluster level, uncorrected.
Acknowledgements
This study was supported by the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2014M3C7A1064752).
References
1. Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, et al. Evolving Evidence for the Value of Neuroimaging Methods and Biological Markers in Subjects Categorized with Subjective Cognitive Decline. J Alzheimers Dis. 2015; 48:Suppl 1. S171–S191.

2. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008; 23:1191–1202.

3. Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:280–292.

4. Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014; 10:844–852.

5. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014; 130:439–451.

6. Kim JM, Stewart R, Shin IS, Choi SK, Yoon JS. Subjective memory impairment, cognitive function and depression--a community study in older Koreans. Dement Geriatr Cogn Disord. 2003; 15:218–225.

7. van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, et al. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013; 9:481–487.

8. Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006; 27:1751–1756.

9. Hong YJ, Yoon B, Shim YS, Ahn KJ, Yang DW, Lee JH. Gray and White Matter Degenerations in Subjective Memory Impairment: Comparisons with Normal Controls and Mild Cognitive Impairment. J Korean Med Sci. 2015; 30:1652–1658.

10. Hong JY, Yun HJ, Sunwoo MK, Ham JH, Lee JM, Sohn YH, et al. Cognitive and cortical thinning patterns of subjective cognitive decline in patients with and without Parkinson's disease. Parkinsonism Relat Disord. 2014; 20:999–1003.

11. Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008; 63:609–618.

12. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010; 25:1071–1076.

13. Christensen KJ, Multhaup KS, Nordstrom S, Voss K. A cognitive battery for dementia: development and measurement characteristics. J Consult Clin Psychol. 1991; 3:168–174.

14. Bae JN, Cho MJ. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J Psychosom Res. 2004; 57:297–305.

15. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356.

16. Peter J, Scheef L, Abdulkadir A, Boecker H, Heneka M, Wagner M, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement. 2014; 10:99–108.

17. Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009; 63:178–188.

18. Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010; 6:67–77.

19. Chhatwal JP, Schultz AP, Johnson K, Benzinger TL, Jack C Jr, Ances BM, et al. Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology. 2013; 81:736–744.

20. Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, et al. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009; 457:66–70.

21. Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry. 2007; 22:387–394.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download