Abstract
Sirtuins (SIRTs) are involved in multiple cellular processes. And they are involved in cellular path-ways related aging, cancer, and a variety of cellular functions including cell cycle, DNA repair and proliferation. Also they modulate life span. Stem cells have the ability to self-renew for unlimited proliferation and differentiate into various cell types. It has been a little known that the mutation of undifferentiated stem cells in tissue may re-sult in the development of cancer cells by genotoxic carcinogens. Therefore, this study investigated whether some carcinogenic compounds can modulate the expression of sirtuin mRNA on human adipose-derived stem cells.
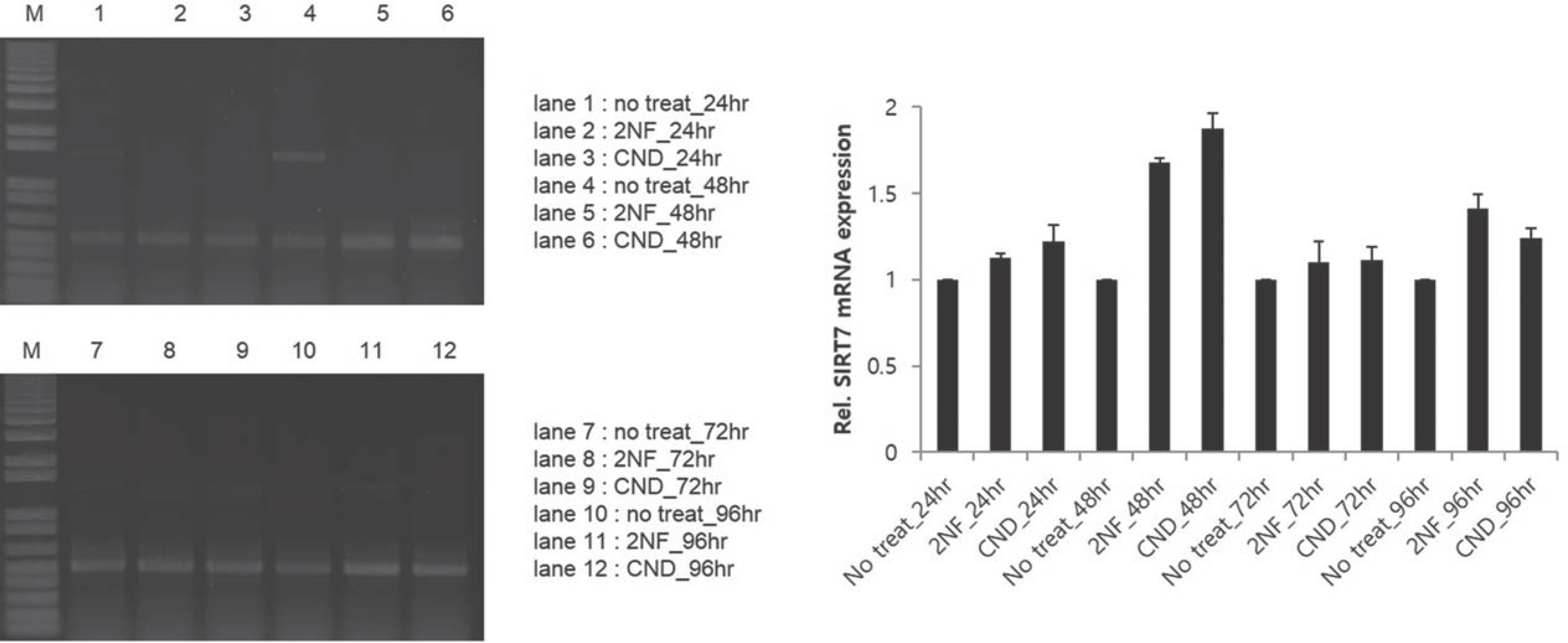
Adipose-derived stem cells(ADSC) were exposed to genotoxic carcinogenic compound(2-nitrofluorene, 2NF) and non-genotoxic carcinogenic compound(clonidine, CND) for 24 hours, 48 hours, 72 hours and 96 hours. The expression of SIRT1 mRNA increased on 72 hours. Expressions of SIRT2 and SIRT7 mRNA increased robustly on 48 hours. But all of SIRTs decreased to a level before a treatment of genotoxic compound on adipose-derived stem cells.
These results demonstrated that a treatment of genotoxic compound induced the expression of SIRT mRNA only in the short time. But their level returned to untreated cells on 96 hours. They suggest that the possibility that the sirtuins can retard the carcinogenesis of adipose derived stem cells.
References
1. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13:225–38.

2. Sebastian C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012; 287:42444–52.
3. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999; 13:2570–80.

4. Guarente L, Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011; 364:2235–44.
5. Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwen-burgh C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010; 143:802–12.

6. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010; 12:662–7.

7. Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010; 40:893–904.

9. Martinez-Climent JA, Andreu EJ, Prosper F. Somatic stem cells and the origin of cancer. Clin Transl Oncol. 2006; 8:647–63.
10. Yamashita YM, Fuller MT. Asymmetric stem cell division and function of the niche in the Drosophila male germ line. Int J Hematol. 2005; 82:377–80.

11. Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J Cell Sci. 2005; 118:665–72.

12. Martinez-Pastor B, Mostoslavsky R. Sirtuins, metabolism, and cancer. Front Pharmacol. 2012; 3:22.

13. Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW, et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011; 71:4138–49.

14. Choi HN, Bae JS, Jamiyandorj U, Noh SJ, Park HS, Jang KY, et al. Expression and role of SIRT1 in hepatocellular carcinoma. Oncol Rep. 2011; 26:503–10.
15. Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Go-ren A, Zhong L, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012; 151:1185–99.
16. Van Meter M, Mao Z, Gorbunova V, Seluanov A. SIRT6 overexpression induces massive apoptosis in cancer cells but not in normal cells. Cell Cycle. 2011; 10:3153–8.

17. Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011; 20:487–99.

18. Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012; 487:114–8.

19. Doktorova TY, Ellinger-Ziegelbauer H, Vinken M, Vanha-ecke T, van Delft J, Kleinjans J, et al. Comparison of geno-toxicant-modified transcriptomic responses in conventional and epigenetically stabilized primary rat hepatocytes with in vivo rat liver data. Arch Toxicol. 2012; 86:1703–15.

20. Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011; 19:416–28.
21. Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011; 30:2986–96.
22. Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. SIRT3 is a mitochondria-lo-calized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010; 17:41–52.

23. Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-medi-ated acetylation of histone H3 on lysine 56. Nature. 2009; 459:113–7.

24. Dearfield KL, Thybaud V, Cimino MC, Custer L, Czich A, Harvey JS, et al. Follow-up actions from positive results of in vitro genetic toxicity testing. Environ Mol Mutagen. 2011; 52:177–204.

25. Rothfuss A, Honma M, Czich A, Aardema MJ, Burlinson B, Galloway S, et al. Improvement of in vivo genotoxicity assessment: combination of acute tests and integration into standard toxicity testing. Mutat Res. 2011; 723:108–20.

26. Hernandez LG, van Steeg H, Luijten M, van Benthem J. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat Res. 2009; 682:94–109.
Fig. 1.
Morphology of cultured human adipose tissue-derived stem cells (hADSCs) is changed by treatment with carcinogenic compund, 2NF(78 μ M) and CND(600 μ M) for 24 hours, 48 hours, and 72 hours(×100).

Fig. 2.
Growth rates of hADSC are decreased prominently by treat-ment with 2NF(78 μ M) for 24 hours, 48 hours, and 72 hours.
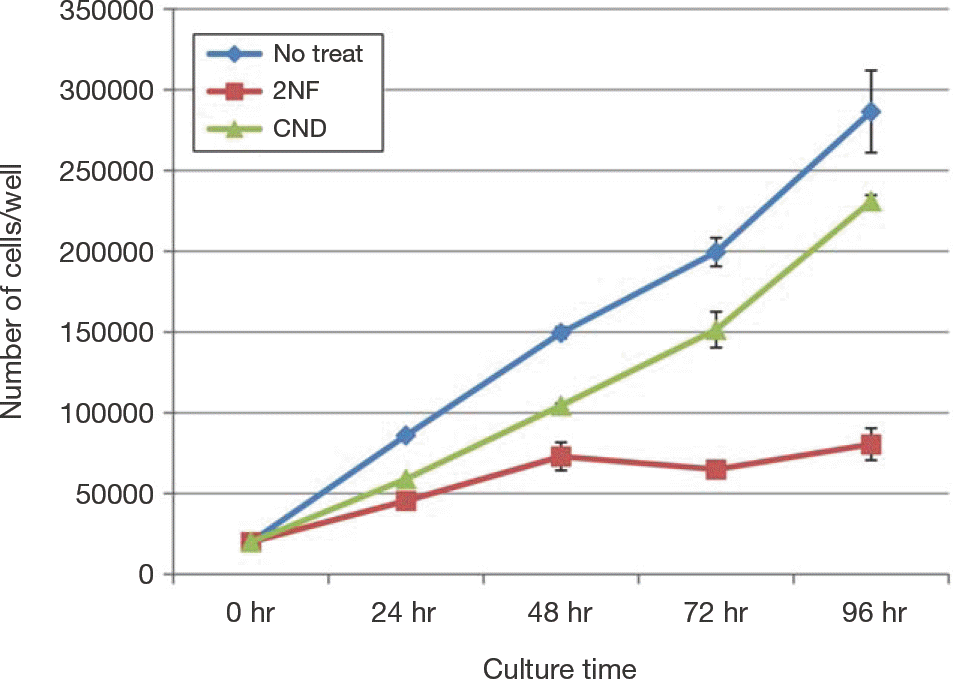
Fig. 3.
Representative gel electrophoresis and analysis for determining sirtuin1 mRNA expression show increased levels in hADSC with treatment of carcinogenic compounds for 72 hours.
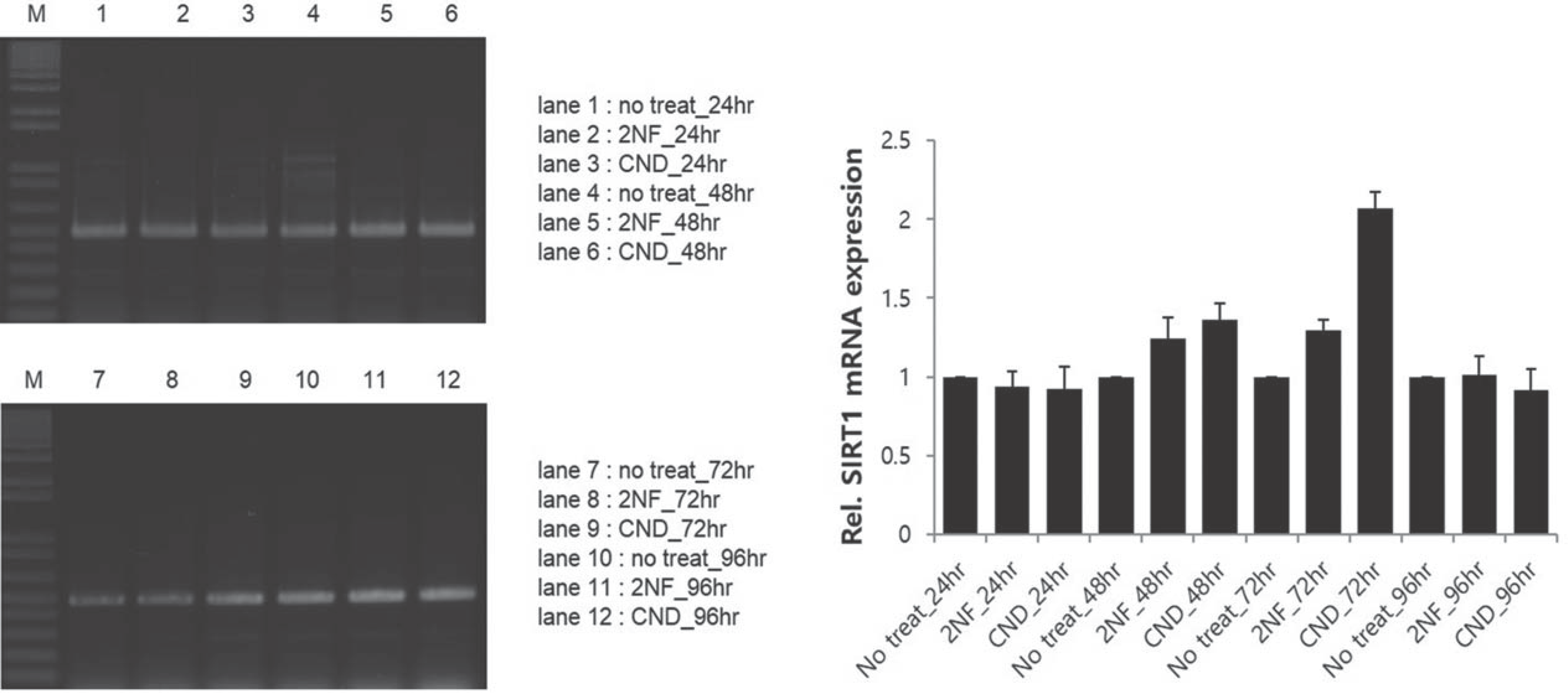
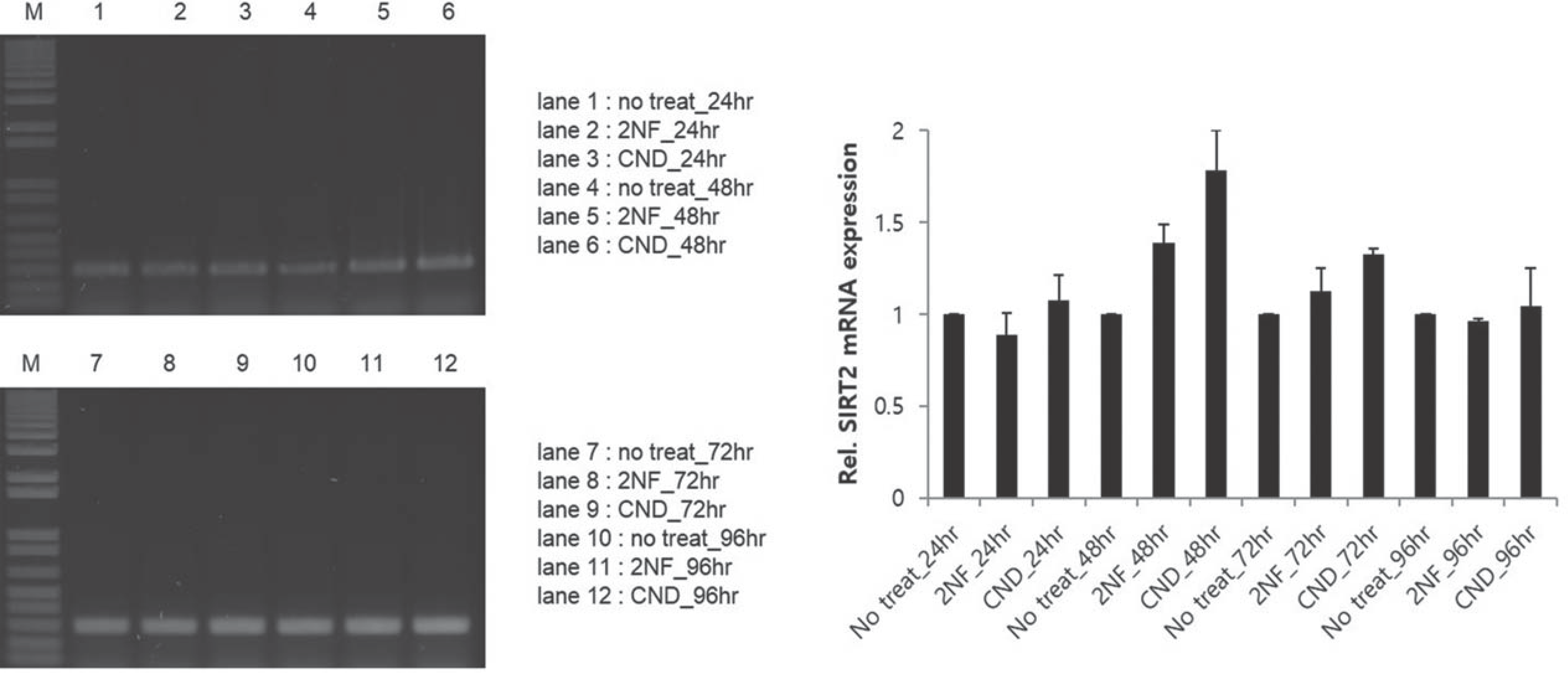
Fig. 4.
Representative gel electrophoresis and analysis for determining sirtuin2 mRNA expression show increased levels in hADSC with treatment of 2NF and CND for 48 hours.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download