Abstract
Background
Methods
Results
Conclusions
ACKNOWLEDGEMENTS
Notes
REFERENCES
Fig. 1
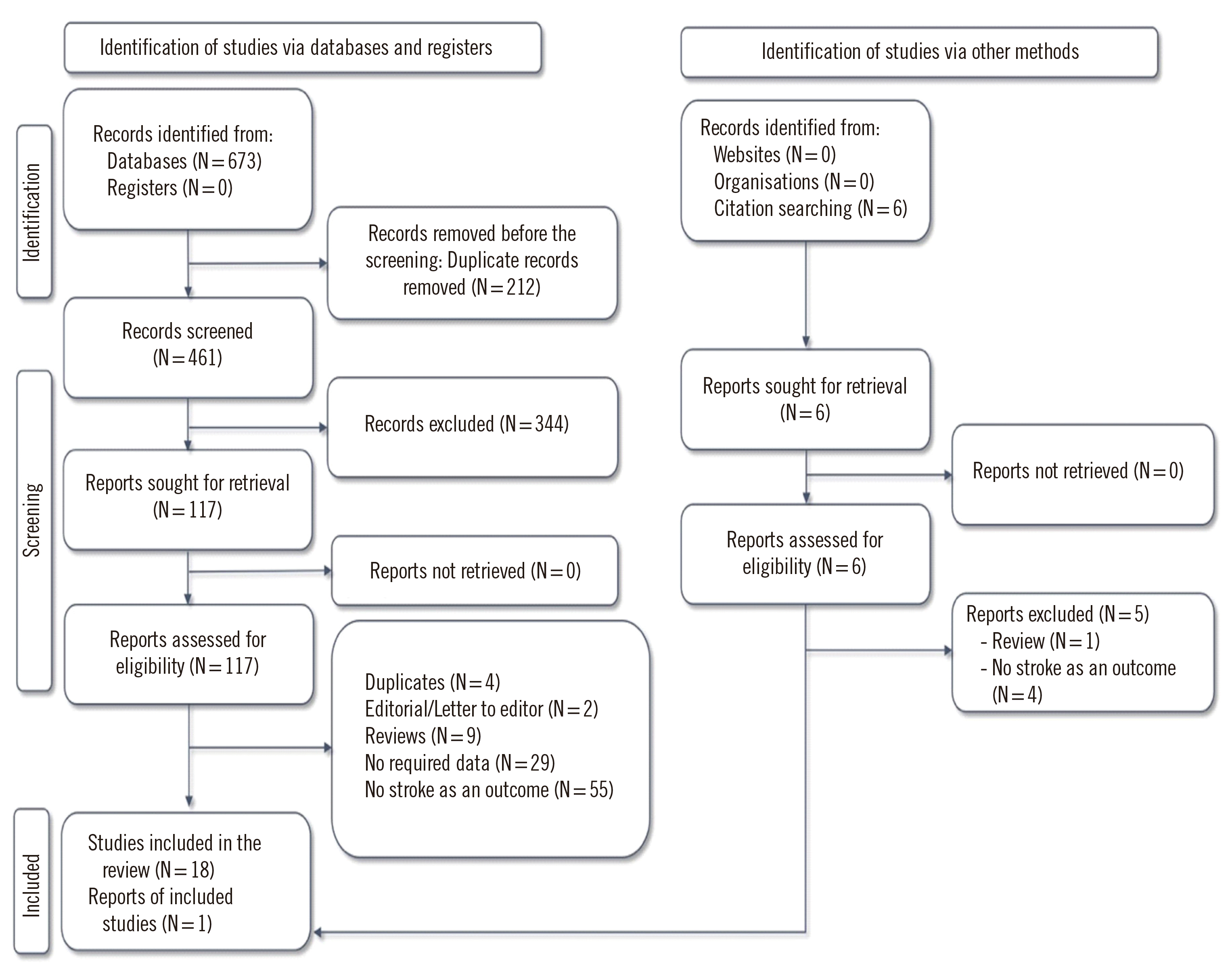
Table 1
| Reference | Design | Patient setting | Sample size | Mean age, yr* | Number of male patients | Follow-up period | Outcome | N outcomes | sST-2 cutoff | Effect size (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Andersson, 2015 [12] | PCS | Healthy subjects | 2,741 | 59 ± 9.7 | Not determined | 11.8 ± 3 yr | Stroke/TIA | 161 |
Q1 Q2 Q3 Q4 Continuous |
Ref. HR = 1.42 (0.85–2.37) HR = 1.73 (1.05–2.84) HR = 1.76 (1.06–2.92) HR = 1.6 (1.01–2.54) |
| Ischemic stroke | 105 |
Q1 Q2 Q3 Q4 Continuous |
Ref. HR = 1.47 (0.77–2.8) HR = 1.82 (0.97–3.41) HR = 1.85 (0.98–3.49) HR = 1.77 (1.01–3.12) |
|||||||
| Bai, 2020 [13] | RCS | Hospitalized patients with CHD | 1,113 | 65 (32–95) | NR | 3.9 yr | Stroke | 49 | Trend over terciles | Effect size: NR P < 0.001 |
| Hammer, 2022 [14] | PCS | Diabetic hemodialysis | 1,196 | 66 ± 8.3 | 645 | 4 yr | Fatal stroke | 94 |
Q1: < 20.1 ng/mL Q2: 20.1–25 Q3: 25.1–32.6 Q4: > 32.6 |
Ref. HR = 0.72 (0.39–1.33) HR = 1.51 (0.85–2.69) HR = 1.92 (1.17–3.14) |
| Hijazi, 2020 [15] | RCT | AF and at least one CHADS2 risk factor | 4,406 | 70.1 | 2,789 | 1.9 yr | Ischemic stroke/SEE | 282 | Third vs. first quartile | HR= 1.232 (1.05–1.446) |
| AF and at least one of the following risk factors: previous stroke or TIA, CHF or reduced LVEF < 40%, age > 75 yr | 1,218 | 72.2 | 612 | Ischemic stroke/SEE | 149 | Third vs. first quartile | HR= 1.02 (0.803–1.296) | |||
| Hughes, 2014 [16] | PCS | Healthy subjects | 7,997 | 48.8 ± 22.1 | 4,225 | 15 yr | Stroke | 354 |
Q1 Q2 Q3 Q4 Continuous |
Ref. HR = 1.31 (0.91–1.89) HR = 1.15 (0.79–1.68) HR = 1.18 (0.82–1.71) HR = 1.03 (0.92–1.16) |
| Khamitova, 2019 [17] | PCS | Acute MI | 180 | 61.4 ± 1.7 | 136 | 1.05 yr | Stroke | 5 | Above normal vs normal | Effect size: NR P = 0.226 |
| Lidgard, 2022 [18] | PCS | Mild to moderate CKD | 2,560 | 56 ± 11.6 | 1305 | 8.13 yr | Stroke | 70 |
Q1: < 11 ng/mL Q2: 11–14.9 Q3: 14.9–20.1 Q4: > 20.1 Continuous |
Ref. HR = 1.59 (0.75–3.36) HR = 1.21 0.56–2.63) HR = 1.43 (0.66–3.11) HR = 1.11 (0.86–1.44) |
| Polineni, 2018 [19] | PCS | CABG/valve replacement | 1,554 | 65.3 | 1,188 | NR | Stroke | NR |
Median T1 T2 T3 |
OR = 3.34 (1.43–7.84) Ref. OR = 6.58 (1.48–29.32) OR = 7.58 (1.43–7.84) |
| Seo, 2019 [20] | PCS | Incident hemodialysis | 182 | 61.3 | 106 | 1.7 yr | Non-fatal stroke | 4 | Median (59.5 ng/mL) | HR = 3.09 (0.32–29.7) |
| Somuncu, 2020 [21] | PCS | Acute MI | 380 | 60.2 | 279 |
1 month 6 months 12 months |
Stroke | 3 | 35 ng/mL |
Effect size: NR P = 0.912 P = 0.052 P = 0.172 |
Abbreviations: PCS, prospective cohort study; RCT, randomized clinical trial; CHD, congestive heart disease; CHADS2, Congestive heart failure, Hypertension, Age ≥ 75 yrs, Diabetes, previous Stroke; AF, atrial fibrillation; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack; MI, myocardial infarction; CKD, chronic kidney disease; CABG, coronary artery bypass graft; SEE, systemic embolic event; NR, not reported; HR, hazard ratio; OR, odds ratio; CI, confidence interval; T, tercile; Q, quartile; Ref., reference.
Table 2
| Reference | Design | Patient setting | Stroke severity* | Sample size | Mean age, yr | Number of male patients | Outcome | Follow-up period | N outcomes | sST-2 cutoff | Effect size (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dieplinger, 2015 [22] | PCS | Ischemic stroke | 3 (3–7) | 721 | 76 (66–84) | 374 | All-cause mortality | 3 months | 81 | > 32 ng/L | RR = 3.77 (2.33–6.57) |
| Lu, 2021 [23] | RCT | Acute ischemic stroke | 4 (3–7) | 635 | 60 ± 10.4 | 445 | Post-stroke depression severity | 3 months | 250 |
Q1: < 117.5 pg/mL Q2: 117.5–162.8 Q3: 162.8–237.7 Q4: > 237.7 Continuous |
Ref. OR = 1.07 (0.65–1.78) OR = 1.22 (0.73–2.02) OR = 1.84 (1.1–3.08) OR = 1.2 (1–1.43) |
| Mechtouff, 2021 [24] | PCS | Acute ischemic stroke | 15 (10–19) | 152 | 69 ± 15 | 90 | All-cause mortality | 12 months | 12 | Above median | HR = 9.9 (1.1–90.3) |
| Sung, 2020 [25] | PCS | Acute ischemic stroke | Mild ( ≤ 8), moderate (9–15), and severe ( ≥ 15) | 99 | 64.4 | 59 | Mild and severe cerebral– cardiac syndrome | In-hospital | NR |
< 14,000 pg/mL ≥ 17,600 pg/mL |
OR= 12.743 (3.836–42.328) OR= 23.448 (2.794–196.801) |
| Tian, 2019 [26] | PCS | TIA/ischemic stroke | 2 (0–4) | 413 | 60.3 ± 11.5 | 285 |
Composite adverse events (ischemic and hemorrhagic stroke, MI, all-cause death) Major disability (mRS 3–6) and death |
12 months |
38 40 |
> 24.61 ng/mL |
HR = 2.517 (1.279–4.956) OR = 3.126 (1.452–6.728) |
| Tian, 2020 [27] | PCS | TIA/ischemic stroke | 2 (0–4) | 430 | 60.2 ± 11.6 | 300 | Mortality/new stroke | 3 months | 19 |
T1: < 11.85 ng/mL T2: 11.85–22.96 T3: > 22.96 Continuous |
Ref. HR = 0.69 (0.11–4.12) HR = 5.14 (1.43–18.51) HR = 1.46 (1.15–1.87) |
| 12 months | 38 |
T1: < 11.85 ng/mL T2: 11.85–22.96 T3: > 22.96 Continuous |
Ref. HR = 1.31 (0.52–3.35) HR = 3 (1.29–6.97) HR = 1.27 (1.03–1.57) |
||||||||
| Wolcott, 2017 [28] | PCS | Acute ischemic stroke | 5 (2–12) | 646 | 69 ± 15 | 346 | Death mRS 3–6 Hemorrhagic transformation | 3 months |
29 84 36 |
NR |
OR = 3.69 (1.54–9.32) OR = 2.97 (1.62–5.52) OR = 5.4 (1.4–35.61) |
| Xu, 2021 [29] | PCS | Acute ischemic stroke | Mild (1–4), moderate (4–15), and severe ( > 16) | 160 | 50.8 | 69 | Depression | In hospital (21 days) | 60 | NR | OR= 0.35 (0.18–0.69) |
| Zhu, 2021 [30] | RCT | Acute ischemic stroke | Median 4–6 | 619 | 60 ± 10.5 | 434 | Cognitive impairment | 3 months | 325 |
Q1: < 117.6 pg/mL Q2: 117.60–163.51 Q3:163.51–238.77 Q4: ≥ 238.77 |
Ref. OR = 1.58 (0.97–2.56) OR = 1.65 (1.01–2.7) OR = 2.38 (1.42–4) |
Abbreviations: PCS, prospective cohort study; RCT, randomized clinical trial; TIA, transient ischemic attack; MI, myocardial infarction; mRS, modified Rankin Scale; RR, relative risk; OR, odds ratio; HR, hazard ratio; NR, not reported; Q, quartile; T, tercile; Ref., reference; IQR, interquartile range.
Table 3
| Reference | Risk of bias | Applicability | Overall | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | ||
| Studies on prediction of stroke | ||||||||
| Andersson, 2015 [12] | Low | Low | Low | Low | Low | Low | Low | Low |
| Bai, 2020 [13] | Low | Low | Low | Low | Low | Low | Low | Low |
| Hammer, 2022 [14] | Low | Low | Low | Low | Low | Low | Low | Low |
| Hijazi, 2020 [15] | Low | Low | Low | Low | Low | Low | Low | Low |
| Hughes, 2014 [16] | Low | Low | Low | Low | Low | Low | Low | Low |
| Khamitova, 2019 [17] | Unclear | Low | Unclear | Low | Low | Low | Low | Some concern |
| Lidgard, 2022 [18] | Low | Low | Unclear | Low | Low | Low | Low | Some concern |
| Polineni, 2018 [19] | Low | Low | Low | Unclear | Low | Low | Low | Some concern |
| Seo, 2018 [20] | Low | Low | Low | Low | Low | Low | Low | Low |
| Somuncu, 2020 [21] | Unclear | Low | Unclear | Low | Low | Low | Low | Some concern |
| Studies on prognosis of stroke | ||||||||
| Dieplinger, 2015 [22] | Unclear | Low | Low | Low | Low | Low | Low | Some concern |
| Lu, 2021 [23] | Low | Low | Low | Low | Low | Low | Low | Low |
| Mechtouff, 2021 [24] | Low | Low | Low | Low | Low | Low | Low | Low |
| Sung, 2020 [25] | Low | Low | Low | Low | Low | Low | Low | Low |
| Tian, 2019 [26] | Low | Low | Low | Low | Low | Low | Low | Low |
| Tian, 2020 [27] | Low | Low | Low | Low | Low | Low | Low | Low |
| Wolcott, 2017 [28] | Low | Low | Low | Low | Low | Low | Low | Low |
| Xu, 2021 [29] | Unclear | Low | Low | High | Low | Low | Low | Some concern |
| Zhu, 2021 [30] | Low | Low | Low | Low | Low | Low | Low | Low |
Table 4
| Outcome | Sample size Follow-up time | Risk of bias | Heterogeneity (I2 value) | Indirectness | Imprecision | Publication bias | Quality of evidence* |
|---|---|---|---|---|---|---|---|
| Stroke |
23,527 1–15 years |
Not serious |
Serious* (Q1: Ref. Q2: 0.00% Q3: 0.00% Q4: 80.76%) |
Not serious | Not serious | Not present |
Low ⊕⊕◯◯ Rated down one score • Possible heterogeneity Rated up one score • Possible dose-response gradient |
| Post-stroke mortality |
1,519 3–12 months |
Not serious | Not serious (0.00%) | Not serious | Serious | Not present |
Moderate ⊕⊕⊕◯ Rated down one score • Imprecision (wide CIs) Rated up two scores • Possible dose-response gradient •Large effect size ( > 2) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download