Abstract
Objective
We evaluated the usefulness of human epididymis protein 4 (HE4), a tumor marker, during and after treatment in patients with ovarian cancer (OC).
Methods
We included Japanese patients newly diagnosed with OC treated at the National Cancer Center Hospital between 2014 and 2021. The HE4 levels were measured in the serum stored during diagnosis. To evaluate the concordance between HE4 and the imaging results, we employed sequential pairs of blood sampling points and the results of imaging examinations. We compared the timing of the elevated HE4 levels, imaging diagnoses, and elevated cancer antigen 125 (CA125) levels in patients with recurrence. The Ethics Review Committee of our institution (2021-056) reviewed this study.
Results
Forty-eight patients with epithelial OC were eligible for enrollment. The sensitivity, specificity, and positive and negative predictive values of HE4 (criterion, 70 pmol/L) for disease progression during the follow-up period were 79.4%, 59.1%, 32.5%, and 92.0%, respectively (time point, n=317). We evaluated the relationship between HE4 and CA125 variability and disease status (recurrence or no recurrence). For recurrence, the sensitivity and negative predictive value of HE4 (criterion, 70 pmol/L), CA125 (criterion, 35 U/mL), and combination of HE4 and CA125 were 77.8%, 85.2%, and 92.6% and 75.0%, 82.6%, and 88.9%, respectively (n=48). Among the 27 patients who exhibited recurrence, 16 and nine showed earlier increased HE4 levels than the relevant imaging and CA125 levels, respectively.
Ovarian cancer (OC) is a common malignant tumor in females. In the United States, cancer of the ovaries, fallopian tubes, and peritoneum is the fifth most common cause of cancer-related deaths among female patients [1]. In 2020, an estimated 313,959 individuals worldwide were diagnosed with OC, and 207,252 individuals died from OC [1]. In Japan, the incidence of OC in 2021 was projected to reach 13,100, and 4,700 OC deaths were projected in the same year [2]. The number of OC patients in Korea has reportedly increased in recent years [3].
Human epididymis protein 4 (HE4) was first identified in the epithelium of the distal epididymis [4]. HE4 is expressed in the respiratory epithelium, normal cells of the reproductive and other tissues, and malignant ovarian tissues [5–7]. Furthermore, high HE4 levels have been documented in the serum of patients with ovarian malignancies, and several meta-analyses have reported the performance of serum HE4 as a diagnostic biomarker of OC. In two meta-analyses conducted by Zhen et al. [8] (25 studies) and Wang et al. [9] (28 studies), serum HE4 demonstrated pooled sensitivity, specificity, and area under the curve (AUC) values of 0.74/0.76, 0.90/0.94, and 0.89/0.89, respectively, whereas cancer antigen 125 (CA125) had pooled sensitivity, specificity, and AUC values of 0.74/0.79, 0.83/0.82, and 0.85/0.87, respectively. In another meta-analysis by Yang et al. [10], which examined 31 studies, serum HE4 yielded pooled sensitivity, specificity, and AUC values of 0.74, 0.89, and 0.96, respectively. These results suggest that serum HE4 exhibits high specificity and moderate sensitivity for diagnosing OC and can potentially diagnose OC along with the CA125 levels. Furthermore, it has been reported that HE4 does not significantly increase in benign gynecological conditions, including benign tumors [11,12], whereas the CA125 levels are known to be elevated in common benign conditions such as endometriosis and fibroids [13]. In a Japanese clinical study by Fujiwara et al. [14], HE4 had a diagnostic performance equal to or greater than that of CA125, and the HE4 value did not increase substantially under benign conditions and showed no significant correlation with CA125. Furthermore, the authors reported that the risk of ovarian malignancy algorithm, which combines HE4 with CA125 and menopausal information, can provide better discrimination between benign and malignant tumors than either HE4 or CA125 alone [14]. Capriglione et al. [15] discussed the latest evidence reported in seven studies on the use of HE4 to monitor OC treatment and detect OC recurrence, and HE4 is considered a promising tool for OC recurrence; however, the number of studies evaluating HE4 during OC follow-up remains limited.
In Japan, tumor markers are listed as a recommended posttreatment follow-up diagnostic test in the 2020 edition of the “guidelines for treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer” [16]. For detecting recurrence, as a general rule, bilateral salpingo-oophorectomy is performed after treatment, and a normal range of CA125 of ≤15 to 20 U/mL has been suggested, similar to that in postmenopausal females [17]. However, the guidelines state that early diagnosis can be attempted based on gradual changes in the CA125 levels rather than absolute values [16]. Given that the optimal criteria for changes in observed CA125 values are not clearly defined, they may need to be considered for the HE4 levels.
Herein, we conducted a retrospective clinical study assessing Japanese patients with OC in an exploratory manner to evaluate the follow-up detection of the tumor marker HE4.
Following the Japanese law, an opt-out model of consent was implemented instead of reinformed consent for blood samples and patient information previously collected for use in certain forms of future research. The study protocol was approved by the Independent Ethics Committee of the National Cancer Center Research Ethics Review Board (research subject number: 2021-056). Of the serum specimens stored at −20°C at the National Cancer Center Hospital, 809 specimens from 61 patients collected between March 10, 2014, and February 16, 2021, were used in this study. Among these patients, we selected female patients aged ≥20 years treated for OC whose pretreatment HE4 values were >70 pmol/L if premenopausal or 140 pmol/L if postmenopausal.
This study used ARCHITECT HE4 and ARCHITECT CA125 II (Abbott Laboratories, Abbott Park, IL, USA). The samples were thawed, inverted, mixed immediately before use, and centrifuged at 4°C and 2,000×g for 50 minutes. The samples were analyzed in accordance with the package inserts. If a sufficient sample amount was available and the sample was over the measurement range, automatic or manual dilution was performed according to the package insert, and the diluted sample was measured again.
For optimal criteria to perform follow-up using the HE4 levels, we established certain criteria candidates (i.e., 70 pmol/L, 140 pmol/L, 25% elevation from the previous value, 14% elevation from the previous value, twofold elevation from the lowest value, or three consecutive elevations) and conducted the study in an exploratory manner.
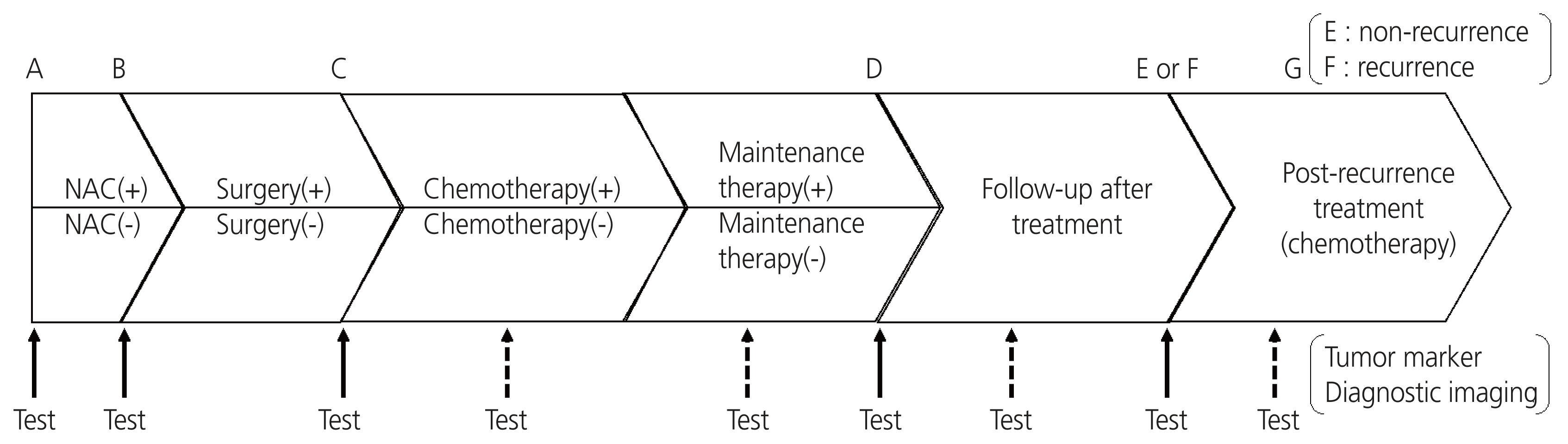
Each sequential pair point was divided into sections, as shown in Fig. 1. The sections were categorized according to the following definitions: the allowable difference between the imaging test date and blood sampling date was determined within 45 days before and after the imaging test; the blood sampling test performed within 45 days after surgery was determined as the postsurgical test (C); the effect of chemotherapy (including neoadjuvant chemotherapy and maintenance therapy) was limited to 1 month after the end of chemotherapy, and when it spanned multiple sections from A to G, it was evaluated as described in the previous section. At each sequential pair point with valid diagnostic imaging results, treatment efficacy was judged using the response evaluation criteria in solid tumors guidelines (version 1.1) [18] and classified into four categories based on the overall response: response, stable, progressive (including recurrence), or not evaluable (excluded from valid sequential pairs). Using the table (m×n contingency table), a 2×2 table of disease states (progression or no progression) and HE4 and/or CA125 values (elevated or not elevated) was created, and the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall agreement rate were calculated. Pearson’s chi-square test and Fisher’s exact test (if there were fewer than five frequencies in each square in the 2×2 table) were implemented using JMP version 16.0.0 (SAS Institute Inc., Cary, NC, USA) to evaluate the association between tumor marker variability and disease state.
Of the 61 patients (809 samples), 48 (698 samples) were analyzed, excluding one who did not receive treatment, nine whose HE4 values did not exceed the reference values (premenopausal, 70 pmol/L; postmenopausal, 140 pmol/L) before treatment, and three with malignant tumors of other organs. The number of blood collection points with valid diagnostic imaging results from before treatment initiation to after the end of recurrence treatment was 317 sequential pairs (excluding five sequential pairs that were “not evaluable” in the treatment efficacy judgment). Considering patient information, the median age was 57 years (range, 42–80), 31 patients were postmenopausal with a pretreatment median HE4 value of 603.1 pmol/L (range, 149.8–7,121.4), 17 patients were premenopausal with a pretreatment median HE4 value of 343.3 pmol/L (range, 75.7–4,056.5), and all were newly diagnosed with epithelial OC. The pretreatment median CA125 value of the 48 patients was 1,362.5 U/mL (range, 40.6–23,877.2), and all pretreatment values for CA125 were greater than the reference value (35 U/mL). Regarding the OC histological subtype, 36 patients had serous (high-grade) tumors, four had endometrioid tumors, six had clear cells, and two had other subtypes. Regarding OC stages, three, two, 25, and 18 patients were at stages I, II, III, and IV, respectively. Additionally, the median observation period was 20.8 months (5.6–43.9 months), and recurrence was confirmed in 27 patients during the observation period.
We evaluated the candidates using four criteria for HE4 (70 pmol/L, 140 pmol/L, 25% elevation from the previous value, and 14% elevation from the previous value) for the entire follow-up period (A–E, A–F, or A–G; Fig. 1). The sensitivity values of 55.6–79.4%, specificity values of 59.1–90.2%, PPVs of 32.5–60.3%, NPVs of 88.1–92.0%, and overall agreement rates of 63.1–84.2% were indicated (Table 1). Considering CA125 (criteria, 35 U/mL), the sensitivity, specificity, PPV, NPV, and overall agreement were 90.5%, 63.4%, 38.0%, 96.4%, and 68.8%, respectively (Table 1). We detected a statistically significant association (P<0.001) between HE4 or CA125 variability and the disease state for all criteria.
We evaluated four criteria for HE4 (twofold elevation from the lowest value, 70 pmol/L, 140 pmol/L, and three consecutive elevations) at the posttreatment follow-up (D–E or D–F; Fig. 1). The sensitivity values of 0–77.8%, specificity values of 75.8–100%, PPVs of 60.3–76.5%, NPVs of 74.1–87.8%, and overall concordance rates of 74.1–77.9% were indicated (Table 1). Considering CA125 (criteria, 35 U/mL), the sensitivity, specificity, PPV, NPV, and overall agreement were 86.7%, 82.1%, 69.6%, 92.9%, and 83.6%, respectively (Table 1). We noted a statistically significant association (P<0.001) between HE4 or CA125 variability and disease state for all criteria, excluding one criterion (three consecutive increases).
We evaluated four criteria candidates for HE4 (70 pmol/L, 140 pmol/L, 25% elevation from the previous value, and 14% elevation from the previous value) at follow-up during drug treatment (A–B, C–D, or F–G; Fig. 1). The sensitivity values of 44.4–83.3%, specificity values of 49.0–96.6%, PPVs of 16.9–61.5%, NPVs of 93.3–96.5%, and overall concordance rates of 52.8–90.8% were indicated (Table 1). Considering CA125 (criteria, 35 U/mL), the sensitivity, specificity, PPV, NPV, and overall agreement were 100.0%, 51.7%, 20.5%, 100.0%, and 57.1%, respectively (Table 1). We detected a statistically significant association (P=0.011 or P<0.001) between HE4 or CA125 variability and the disease state for all criteria.
During drug treatment and posttreatment follow-up, the 70 pmol/L and 140 pmol/L HE4 criteria demonstrated a favorable NPV. We established 70 pmol/L and 140 pmol/L as the criteria for the HE4 levels and 35 U/mL for CA125 and evaluated the diagnosis of patients with recurrence and no recurrence. Based on our established criteria of 70 pmol/L or 140 pmol/L for HE4, we obtained a sensitivity of 77.8% or 44.4%, specificity of 85.7% or 100.0%, PPV of 87.5% or 100.0%, NPV of 75.0% or 58.3%, and overall agreement rate of 81.3% or 68.8%, respectively (Table 1). The HE4 criterion of 70 pmol/L showed superior sensitivity and NPV compared with 140 pmol/L for the diagnosis of recurrence or no recurrence. Therefore, the HE4 criterion of 70 pmol/L was used in subsequent analyses. Considering the 35 U/mL criteria for CA125, the sensitivity, specificity, PPV, NPV, and overall agreement rate were 85.2%, 90.5%, 92.0%, 82.6%, and 87.5%, respectively (Table 1). Considering either the 35 U/mL criteria for CA125 or the 70 pmol/L criteria for HE4, the sensitivity, specificity, PPV, NPV, and overall agreement rate were 92.6%, 76.2%, 83.3%, 88.9%, and 85.4%, respectively (Table 1). We observed a statistically significant association (P<0.001) between HE4 or CA125 variability and the disease state for all criteria.
Gradual changes in the HE4 values were plotted along with imaging diagnosis and CA125 values (Figs. 2, 3 and Supplementary Figs. S1–S46). In 27 patients with recurrence, HE4 alone, CA125 alone, and HE4 and/or CA125 levels were elevated earlier than the imaging diagnosis in 59% (16/27), 41% (11/27), and 67% (18/27) of patients, respectively. In contrast, imaging diagnosis was made earlier than HE4 alone, CA125 alone, and HE4 and/or CA125 in 22% (6/27), 15% (4/27), and 7% (2/27) of patients, respectively (Table 2). Furthermore, elevated HE4 levels earlier than CA125 levels were observed in 33% (9/27) of patients, and elevated CA125 levels earlier than HE4 levels or elevated CA125 levels alone were observed in 30% (8/27) (Table 3). In one patient, the values did not exceed the established criteria (HE4, 70 pmol/L; CA125, 35 U/mL). Conversely, of the 21 patients with no recurrence, 18 had values below the HE4 criterion (70 pmol/L) after treatment until the end of the observation period. Of the three patients, imaging diagnosis 1 month after the study period confirmed recurrence in one patient whose posttreatment HE4 values were above the established criterion (70 pmol/L) (Supplementary Fig. S44).
This study evaluated the clinical usefulness of the tumor marker HE4 in the follow-up of patients with OC. We analyzed 48 patients, with a median age of 57 years (range, 42–80) and median observation period of 20.8 months (range, 5.6–43.9), comprising 31 postmenopausal and 17 premenopausal patients, 27 patients with recurrence, and 21 patients with no recurrence. Furthermore, this study reflected the diverse clinical statuses of OC, including OC histological subtypes of serous (high-grade), endometrioid, and clear cell and OC stages I, II, III, and IV.
During the entire follow-up assessment period, fixed values of 70 and 140 pmol/L and increases of 25% and 14% were used as candidate HE4 criteria. The relationship between HE4 variability and disease state (progression or no progression) was examined at each sequential pair point with diagnostic imaging results. Accordingly, we observed a statistically significant association between HE4 variability and disease state for all criteria, excluding one criterion (three consecutive elevations), during the entire follow-up, follow-up during treatment, and follow-up after treatment. Given that only 27 sequential pair points could be used as the criterion for three consecutive elevations, the number of cases was deemed insufficient for statistical analysis. In addition, we detected a statistically significant association between CA125 (criterion, 35 U/mL) variability and disease state for all follow-up periods. Therefore, HE4 could be useful during the follow-up of OC treatment, given that variabilities in HE4 values were consistent with the imaging judgment or the clinician’s judgment of the disease, similar to the variabilities in the CA125 levels. Considering the use of tumor markers for follow-up, it is appropriate to focus on ensuring that the markers facilitate imaging diagnosis, signs of recurrence are not missed, and the markers are reliable when negative. Among the candidate criteria employed in this study, 70 pmol/L for HE4, which showed a sensitivity of 79.4% and an NPV of 92.0% during the entire follow-up, can be recommended as the HE4 variation criterion for the follow-up of OC treatment.
In addition, the relationship between HE4 variability and disease state (recurrence or no recurrence) was examined for CA125. We noted a statistically significant association between HE4 and CA125 variability and disease status. HE4 showed a sensitivity and NPV similar to those of CA125, and the combination of HE4 and CA125 revealed better sensitivity and NPV than HE4 or CA125 alone. In the recurrence group, HE4 exceeded the criterion (70 pmol/L) in two patients whose CA125 levels did not exceed the established criterion (35 U/mL) at the time of recurrence, and CA125 exceeded the established criterion (35 U/mL) in four patients whose HE4 levels did not exceed the criterion (70 pmol/L) at the time of recurrence. In addition, the criteria for HE4 (70 pmol/L) and CA125 (35 U/mL) did not exceed in two patients at the time of recurrence. Following a study by Plotti et al. [19], seven of eight patients who had HE4 values of ≥70 pmol/L and CA125 values of ≤35.2 U/mL before surgical intervention exhibited recurrence during the observation period, providing HE4 and CA125 values of ≥83.11 pmol/L and ≤31 U/mL, respectively. The authors suggested that HE4 is useful for the follow-up of patients with OC who are HE4 positive and CA125 negative before surgery. However, we observed that if HE4 and CA125 were positive before treatment, these markers could be used complementarily in follow-up assessments.
HE4, CA125, treatment (chemotherapy or surgery), and disease state (recurrence or no recurrence) were plotted for each patient, and the relationships between tumor marker variability, treatment, and disease state were examined. In 27 patients with recurrence, HE4 alone, CA125 alone, and HE4 and/or CA125 levels were elevated earlier than the imaging diagnosis in 59%, 41%, and 67% of patients, respectively. In contrast, imaging diagnosis was detected earlier than HE4 alone, CA125 alone, and HE4 and/or CA125 in 22%, 15%, and 7% of patients, respectively. Therefore, HE4 may be capable of detecting recurrence earlier than imaging when combined with CA125. In addition, the HE4 level was elevated earlier than the CA125 level in 33% of patients, suggesting that HE4 is superior to CA125 during the follow-up of certain cases.
In contrast, among the 21 patients without recurrence, 18 met the criterion (70 pmol/L) after treatment, and the status continued from after treatment until the end of the observation period. Although three patients continued to exhibit HE4 values above the established criterion (70 pmol/L) after treatment, all were considered to be affected by mild renal dysfunction (serum creatinine level, approximately 0.8–1.3 mg/dL; estimated glomerular filtration rate, 30–60 mL/min/1.73 m2) (Supplementary Figs. S29, S39, S44) [20,21]. Imaging diagnosis 1 month after the study period confirmed recurrence in one of the three patients (Supplementary Fig. S44). In patients with renal dysfunction, careful attention should be paid to the fixed HE4 value and trends in HE4 values.
Schummer et al. [22] reported that 20 of 23 female patients developed OC recurrence and the HE4 level was elevated alone or earlier than the CA125 level in 35% (7/20) of patients at the time of recurrence. However, the follow-up study did not evaluate patients without recurrence [21]. Manganaro et al. [23] enrolled 21 patients with advanced OC who underwent surgery and adjuvant chemotherapy and collected three serum samples at time intervals I (1–3 months after surgery), II (4–6 months after surgery), and III (7–10 months after surgery). Accordingly, in nine patients with relapsed OC, increased HE4 values (criterion, 150 pmol/L) were detected in 89% of patients. In contrast, increased CA125 levels (threshold, 35 U/mL) were documented in 44% of patients at time interval III. In 12 patients with non-relapsed OC, increased HE4 levels were not observed at any time interval. However, increased CA125 levels were documented in six patients [23]. Hatzipetros et al. [24] examined the changes in tumor markers in 13 patients with epithelial OC who underwent radical surgery and received six consecutive cycles of first-line chemotherapy. The serum CA125 and HE4 levels significantly decreased after chemotherapy, which was consistent with the decrease in lesion size detected after chemotherapy [23]. Innao et al. [25] conducted a follow-up study assessing 47 patients with epithelial OC and documented 23 patients with recurrence and 24 patients with no recurrence. On defining the twofold increase in the CA125 or HE4 level after surgery as “rising,” the sensitivity and specificity of HE4 were 91.3 and 87.5% against recurrence, respectively [25]. Our study indicates that HE4 could be a valuable tumor marker during follow-up, similar to previous reports. In addition, our study was conducted on a large scale with numerous evaluation points that simultaneously encompassed both imaging and tumor marker values.
Conversely, the other five patients showed fluctuations (elevation above the criterion of 35 U/mL) in the CA125 values deviating from the pathological transition, which were attributed to lymphatic or ascitic fluid retention after surgery (Supplementary Figs. S3, S15, S16, S19, S38) [26,27]. In contrast, the HE4 values in all patients did not exceed the established criterion (70 pmol/L).
Following the 2020 edition of the Japan Society of Gynecologic Oncology guidelines [16] regarding the use of CA125 in follow-up, attempts have been made to reach an early diagnosis based on gradual changes over time rather than absolute values. Rustin et al. [28] showed that doubling CA125 levels could predict OC progression with a sensitivity of 94%. Herein, we observed a significant association between HE4 variability and disease state, considering the criteria candidates of 25% elevation from previous values, 14% elevation from previous values, and doubling elevation from the lowest HE4 value.
These results suggest that a significant association exists between HE4 variability, treatment, and disease state and that HE4 demonstrates an almost equivalent performance to the existing marker CA125 at follow-up. Moreover, HE4 can be used as a complement to the CA125 levels. Although HE4 was used at a cutoff value of 70 pmol/L for premenopausal females and 140 pmol/L for postmenopausal females to diagnose OC, it is recommended that HE4 be used at a criterion of 70 pmol/L, regardless of premenopausal or postmenopausal status, in the follow-up of OC. In addition, during follow-up treatment, it is necessary to consider the state of renal function against HE4 and the presence of inflammation against CA125.
In conclusion, during follow-up, either during or after OC treatment in Japanese patients, changes in the values of HE4, a tumor marker, were consistent with imaging findings or clinical determination of the disease state. Thus, HE4 could be employed for follow-up assessment during and after OC treatment and may play a complementary role to CA125 during follow-up observations.
Notes
Ethical approval
The study protocol was approved by the Independent Ethics Committee of the National Cancer Center Research Ethics Review Board (research subject number: 2021-056).
References
1. American Society of Clinical Oncology. Ovarian, fallopian tube, and peritoneal cancer: statistics [Internet]. Alexandria: American Cancer Society;c2022. [cited 2022 Apr 18]. Available from: https://www.cancer.net/cancer-types/ovarian-fallopian-tube-and-peritoneal-cancer/statistics.
2. National Cancer Center Cancer Information Service. Projection of cancer mortality and incidence in 2021 [Internet]. Tsukiji: National Cancer Center Japan;c2022. [cited 2022 Apr 18]. Available from: https://ganjoho.jp/public/qa_links/report/statistics/2022_en.html.
3. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.

5. Bingle L, Singleton V, Bingle CD. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene. 2002; 21:2768–73.

6. Galgano MT, Hampton GM, Frierson HF Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006; 19:847–53.

7. Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005; 65:2162–9.

8. Zhen S, Bian LH, Chang LL, Gao X. Comparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in ovarian cancer: a meta-analysis. Mol Clin Oncol. 2014; 2:559–66.

9. Wang J, Gao J, Yao H, Wu Z, Wang M, Qi J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol. 2014; 35:6127–38.

10. Yang Z, Wei C, Luo Z, Li L. Clinical value of serum human epididymis protein 4 assay in the diagnosis of ovarian cancer: a meta-analysis. Onco Targets Ther. 2013; 6:957–66.
11. Hertlein L, Stieber P, Kirschenhofer A, Krocker K, Nagel D, Lenhard M, et al. Human epididymis protein 4 (HE4) in benign and malignant diseases. Clin Chem Lab Med. 2012; 50:2181–8.

12. Omer B, Genc S, Takmaz O, Dirican A, Kusku-Kiraz Z, Berkman S, et al. The diagnostic role of human epididymis protein 4 and serum amyloid-A in early-stage endometrial cancer patients. Tumour Biol. 2013; 34:2645–50.

13. Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989; 4:1–12.

14. Fujiwara H, Suzuki M, Takeshima N, Takizawa K, Kimura E, Nakanishi T, et al. Evaluation of human epididymis protein 4 (HE4) and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools of type I and type II epithelial ovarian cancer in Japanese women. Tumour Biol. 2015; 36:1045–53.

15. Capriglione S, Luvero D, Plotti F, Terranova C, Montera R, Scaletta G, et al. Ovarian cancer recurrence and early detection: may HE4 play a key role in this open challenge? A systematic review of literature. Med Oncol. 2017; 34:164.

16. Tokunaga H, Mikami M, Nagase S, Kobayashi Y, Tabata T, Kaneuchi M, et al. The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. J Gynecol Oncol. 2021; 32:e49.

17. Sugiyama T, Nishida T, Komai K, Nishimura H, Yakushiji M, Nishimura H. Comparison of CA 125 assays with abdominopelvic computed tomography and transvaginal ultrasound in monitoring of ovarian cancer. Int J Gynaecol Obstet. 1996; 54:251–6.

18. Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho. 2009; 36:2495–501.
19. Plotti F, Guzzo F, Schirò T, Terranova C, De Cicco Nardone C, Montera R, et al. Role of human epididymis protein 4 (HE4) in detecting recurrence in CA125 negative ovarian cancer patients. Int J Gynecol Cancer. 2019; 29:768–71.
20. Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem. 2011; 57:1534–44.
21. Nagy B Jr, Krasznai ZT, Balla H, Csobán M, Antal-Szalmás P, Hernádi Z, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem. 2012; 49:377–80.
22. Schummer M, Drescher C, Forrest R, Gough S, Thorpe J, Hellström I, et al. Evaluation of ovarian cancer remission markers HE4, MMP7 and mesothelin by comparison to the established marker CA125. Gynecol Oncol. 2012; 125:65–9.
23. Manganaro L, Michienzi S, Vinci V, Falzarano R, Saldari M, Granato T, et al. Serum HE4 levels combined with CE CT imaging improve the management of monitoring women affected by epithelial ovarian cancer. Oncol Rep. 2013; 30:2481–7.
24. Hatzipetros I, Gocze P, Koszegi T, Jaray A, Szereday L, Polgar B, et al. Investigating the clinical potential for 14-3-3 zeta protein to serve as a biomarker for epithelial ovarian cancer. J Ovarian Res. 2013; 6:79.
25. Innao P, Pothisuwan M, Pengsa P. Does human epididymis protein 4 (HE4) have a role in prediction of recurrent epithelial ovarian cancer. Asian Pac J Cancer Prev. 2016; 17:4483–6.
26. Berger Y, Nevler A, Shwaartz C, Lahat E, Zmora O, Gutman M, et al. Elevations of serum CA-125 predict severity of acute appendicitis in males. ANZ J Surg. 2016; 86:260–3.

Fig. 2
Typical graph representing changes in human epididymis protein 4 (HE4) and cancer antigen (CA125) values in patient experiencing recurrence (PA25) during the entire follow-up. The HE4 values are displayed on the log scale on the left vertical axis, and the CA125 values are displayed on the log scale on the right vertical axis. Progressive disease is confirmed after approximately 25 months during the observation period. OS, optimal surgery.
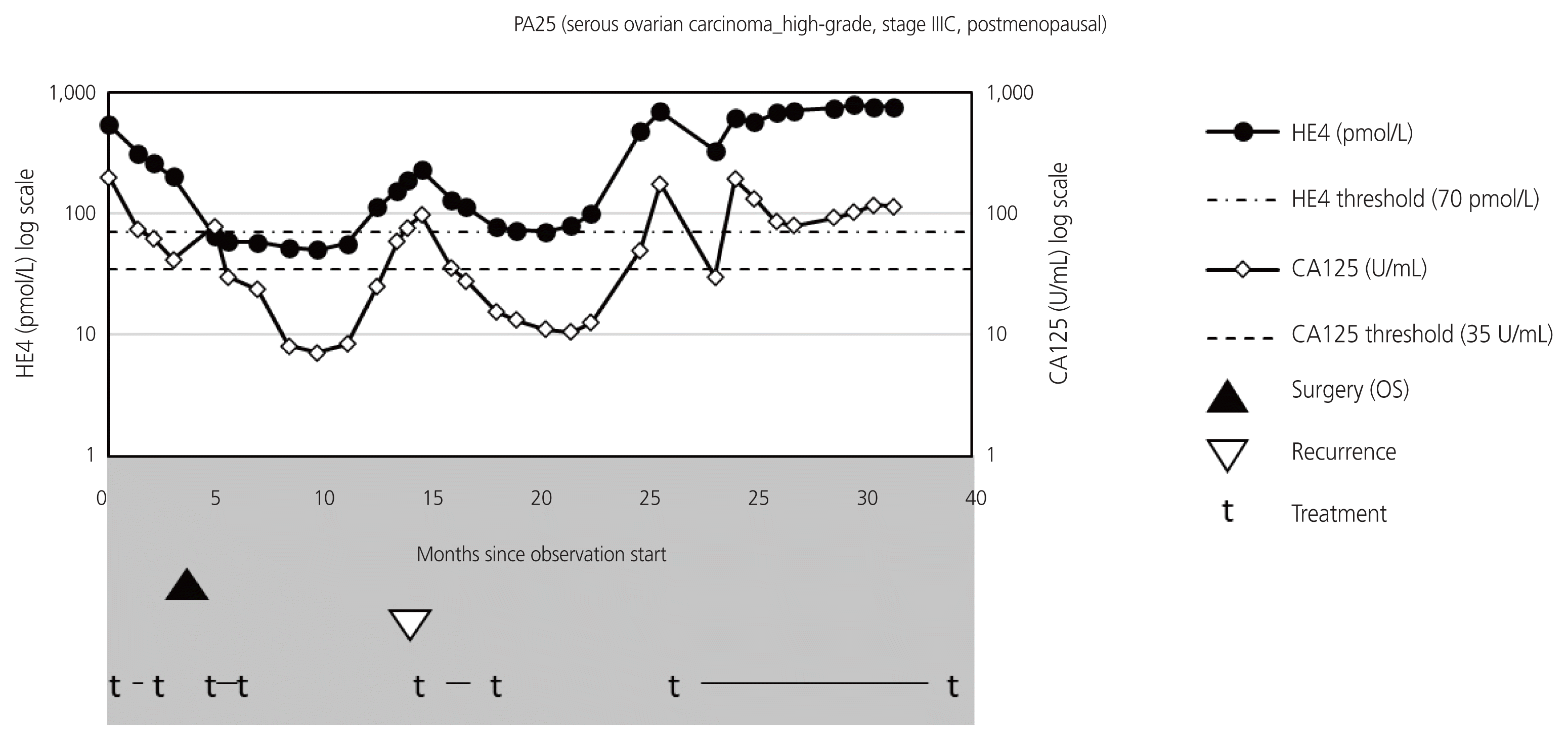
Fig. 3
Graphical representation of patient with recurrence (PA12) shows elevation of the human epididymis protein 4 (HE4) levels earlier than that of the cancer antigen 125 (CA125) levels. The HE4 values are displayed on the log scale on the left vertical axis, and the CA125 values are displayed on the log scale on the right vertical axis. Progressive disease is noted approximately 18 months after starting observation and at the end of the observation period. OS, optimal surgery.
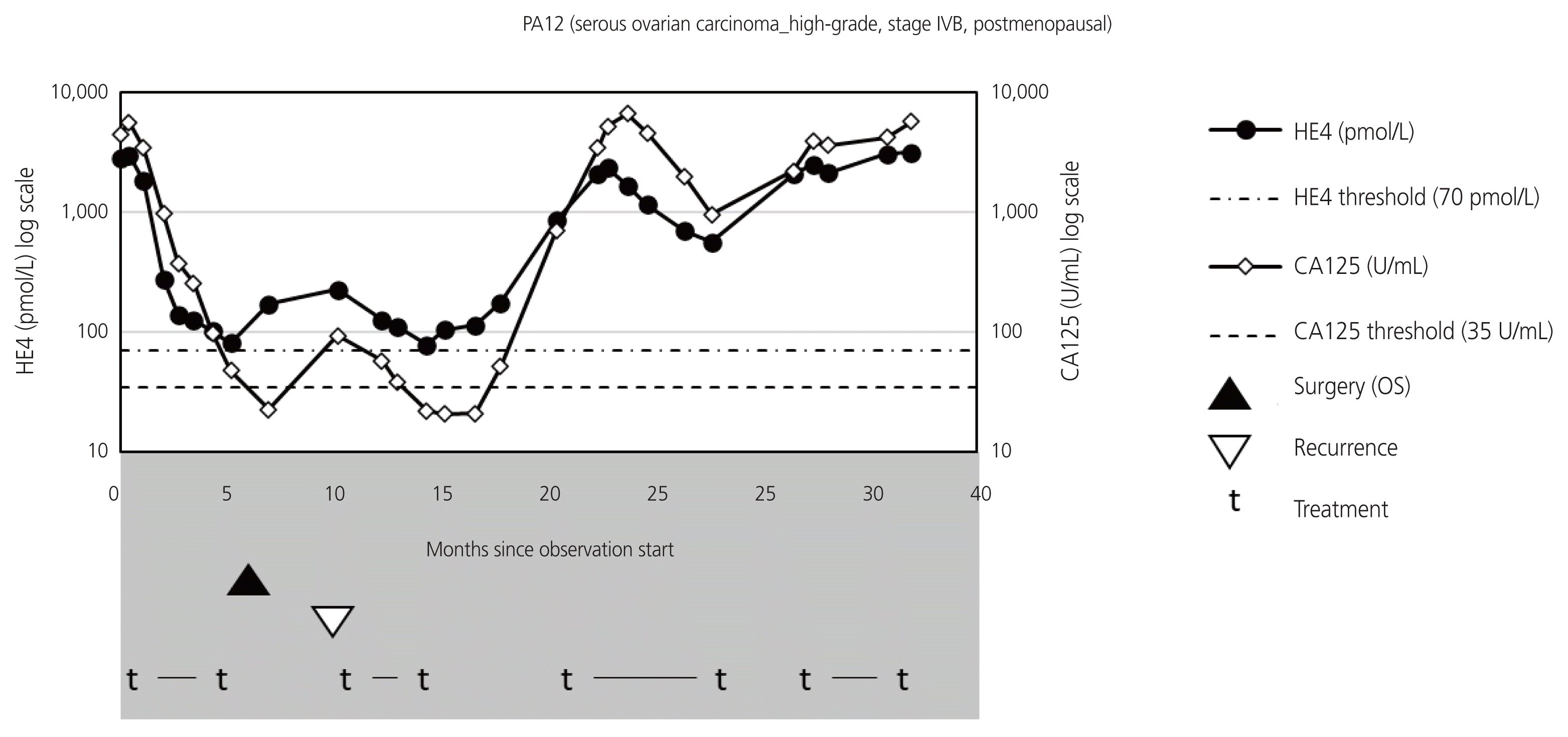
Table 1
Sensitivity, specificity, PPV, NPV, and overall concordance rate against each criterion during each follow-up period for disease progression or recurrence
A–E, A–F, or A–G (entire follow-up); D–E or D–F (posttreatment follow-up); and A–B, C–D, or F–G (follow-up during drug treatment) indicate the assessment sections in the ovarian cancer treatment process (Fig. 1).
Table 2
Number of patients with ovarian cancer recurrence first detected by elevated tumor marker levels compared with recurrence detected using imaging
Table 3
Number of patients with ovarian cancer recurrence first detected by either elevated HE4 or CA125 levels, or both, percent of recurrences detected by each marker
| HE4 alone | HE4 earlier than CA125 | Both at the same time | CA125 earlier than HE4 | CA125 alone | None |
|---|---|---|---|---|---|
| 0 | 9 | 9 | 4 | 4 | 1 |
| 0 (0/27) | 33 (9/27) | 33 (9/27) | 15 (4/27) | 15 (4/27) | 4 (1/27) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download