Abstract
Objective
To investigate the effect of type D personality on cardiac rehabilitation (CR) participation rates and the effect of a short-term CR program.
Methods
Study participants included patients diagnosed with acute coronary syndrome who underwent percutaneous coronary intervention. Patients completed the Type D personality Scale (DS-14) and the Hospital Anxiety and Depression Scale (HADS) at program entry. Subjects were recommended participation in 6 weeks of CR exercise training. Cardiopulmonary exercise test (CPET) was conducted before and after completion of the training. CR participation refers to completion of the 6-week CR exercise program and performance of the secondary CPET. Drop-out refers to the subjects who were unable to participate in the 6-week CR exercise program or to perform the secondary CPET.
Results
At baseline, type D personality was evident in 21 of 63 patients (33.3%). Type D patients were more often depressed (57.1%) and anxious (38.1%) than non-type D patients (31.0% and 9.5%, respectively). At baseline, participants with type D personality showed a decreased body mass index (24.6 vs. 26.1 kg/m2, p=0.025). The type D group displayed a lower CR participation rate (5/21, 23.8%) compared with the non-type D group of (22/42, 52.4%). Logistic regression analysis revealed the association of type D personality with CR drop-out rate (odds ratio=3.87; 95% confidence interval, 1.2–12.5; p<0.05).
Ongoing studies, including studies of psychological risk factors, are designed to identify and reduce risk factors of coronary artery disease (CAD). In the past, the type A behavioral pattern (TABP) was recognized as a CAD risk factor, along with hypertension, smoking, and dyslipidemia. However, a 25-year follow-up study reported that the hostility trait in TABP is not associated with CAD [1].
In the 1990s, the type D personality (TDP), which differs from TABP, was proposed as a risk factor for CAD. Components of TDP include negative affectivity (NA) and social inhibition (SI). NA refers to the tendency to experience distress, regardless of time or place [2]. SI refers to the suppression of negative emotions in social interactions [3].
TDP can affect the prognosis and condition of CAD patients following stenting [3,4]. No reports exist on the effects of TDP on short-term cardiac rehabilitation (CR) or on the CR participation rate in Korea. Accordingly, the present study investigated these topics to determine whether TDP affects the CR participation of CAD patients in Korea, and to raise awareness of TDP.
The participants were patients diagnosed with acute coronary artery syndrome who had undergone percutaneous coronary intervention at the cardiovascular center in our hospital. Most of these patients were referred to the CR clinic on day 2 after the procedure. The patients performed low-intensity (2–3 metabolic equivalents) treadmill exercise to determine the presence of adverse cardiovascular events. Patients were educated on the need for CR and the management of CVD risk factors. The patients were provided with an explanation of the objectives and procedures of the present study and were requested to fill out a voluntary informed consent form. Patients who consented to participate were required to complete the TDP scale and the Hospital Anxiety and Depression Scale (HADS) questionnaire. Patients were excluded if they failed to complete the cardiopulmonary exercise test (CPET) due to underlying problems, such as musculoskeletal or neurological diseases, did not sign the consent form, or did not understand the questionnaire due to mental/cognitive impairment. A schematic flow of the study is provided in Fig. 1.
This was a prospective cohort study. The research protocol, explanation sheets, and consent forms were approved by the Institutional Review Board of Inje University Sanggye Paik Hospital (no. 2015-07-027-001). Patients who consented to participate were required to complete the TDP scale and the HADS questionnaire during hospitalization. The participants’ first outpatient visit occurred within 1–2 weeks of discharge. In this study, CR participation was confirmed in subjects who completed the 6-week CR exercise program and performed the secondary CPET. Drop-out was indicated in subjects who were unable to participate in the 6-week CR exercise program or to perform the secondary CPET.
A CPET was conducted according to the Modified Bruce Protocol. Based on the CPET, the following parameters were measured and recorded: peak heart rate (HRpeak), resting heart rate (HRrest), systolic blood pressure (SBP), diastolic blood pressure (DBP), rate of perceived exertion (RPE), respiratory exchange ratio (RER), oxygen consumption (VO2), and exercise time. The equipment used for the tests included a Q4500 12-channel real-time ECG stress test system (Quinton Instrument, Boston, MA, USA), QMC respiratory gas analyzer (Quinton Instrument), model 412 automatic blood pressure and pulse meter (Quinton Instrument), and a Medtrack ST55 treadmill (Quinton Instrument). The CPET was completed according to the guidelines of the American College of Cardiology and the American Heart Association [5]. The RPE was assessed using a 6–20 Borg scale, with the patients being instructed prior to the test to express their level of exertion during exercise as a numeric value [6].
The Karvonen formula, i.e., (HRpeak-HRrest)×%intensity +HRrest, was used to determine the exercise intensity [7]. With respect to the %intensity of the target heart rate (THR), exercise was performed at 60% intensity in the first 2 weeks, 70% intensity during weeks 3 and 4, and 85% intensity during weeks 5 and 6 [6]. The exercise program consisted of 10 minutes of warm-up exercise (5 minutes stretching followed by 5 minutes light walking), 30 minutes of main exercise (combination of treadmill and bicycle ergometer use), and 10 minutes of cooling down (5 minutes light walking followed by 5 minutes stretching) [8]. Patients with difficulties participating in the hospital-monitored exercise program were trained to perform the exercise on their own, following the same times and procedures. Two patients underwent the home exercise program.
The Korean version of Type D Personality Scale-14 (DS-14) [9] (Supplementary Table S1) was used for the TDP scale, with sufficient internal consistency and construct validity. The TDP scale was developed in Belgium for patients with heart disease and has been used as a tool to assess NA and SI, as components of TDP. The TDP scale consists of the NA and SI subscales. Each subscale contains seven items. Each item is graded on a scale of 0–4 points and the scores from each item are summed. Each subscale has a score range of 0–28 points. If a participant scores ≥10 on both subscales, the participant is classified as having a TDP. A score <10 indicates the absence of TDP [10].
Zigmond and Snaith [11] used anxiety and depression, as generally observed in the hospital environment, to develop the HADS for the measurement of anxiety and depression levels in patients. HADS can be used to assess the level of anxiety and depression in patients admitted to hospital. Standardization and validity of this scale outside Korea has been confirmed. In Korea, a standardization version of HADS [12] has been used to measure anxiety and depression with high validity. The scale, which was used in the present study, consists of 14 items, with 7 odd-numbered items comprising the anxiety subscale (HAD-A) and 7 even-numbered items comprising the depression subscale (HAD-D). Each item is graded on a 4-point scale (0–3 points). The cut-off point for each subscale is 8 points.
The recorded results were statistically analyzed using SAS version 4.2 (SAS Institute Inc., Cary, NC, USA). A x2 test was performed to analyze the inter-group differences of sex; smoking status; prevalence of diabetes, hypertension, and dyslipidemia; percentage of angina pectoris and myocardial infarction; and number of occluded coronary arteries. The paired t-test was performed to analyze within-group differences in body mass index (BMI), left ventricular ejection fraction (LVEF), and age. Mean and standard deviation for all items from the CPET were calculated. The Kolmogorov-Smirnov test and the Shapiro-Wilk test were used to test for the normality of each item. The mean between-group difference for each item was compared using an independent t-test, while the mean within-group difference over time was compared using a paired t-test. However, as the sample size of patients who completed the CR in the TDP group was small and did not show normality, the Wilcoxon rank sum test was used. A repeated-measures ANOVA model was used to examine the group-period interaction of peak oxygen consumption (VO2peak). A linear mixed model test was performed to examine the group-period interaction of VO2peak adjusted for various demographic characteristics. The statistical significance level was set to p<0.05.
A total of 63 patients were classified into TDP (n=21) or non-TDP (n=42) groups based on the TDP scale. The group scores for the subscales of TDP scale are shown in Table 1. The TDP group had a BMI of 24.6 kg/m2, which was significantly lower than in the non-TDP group BMI (24.6 vs. 26.1 kg/m2; p=0.025). No other differences were observed between the two groups in terms of clinical characteristics, including age; sex-ratio; percentage of unstable angina and myocardial infarction; left ventricle ejection fraction (LVEF); prevalence of diabetes, hypertension, and dyslipidemia; and smoking status (Table 2).
A total of 63 participants consented and participated in the study. Of these, 27 (42.9%) completed the 6-week CR exercise program, while 36 (55.6%) participants dropped out. The CR participation rate was significantly lower in the TDP group than in the non-TDP group (5/21, 23.8% vs. 22/42, 52.4%; p<0.05). Logistic regression analysis revealed a significant correlation between CR drop-out and TDP (Table 4). Depression, anxiety, BMI, and CR participation rate were not significant correlated, obviating the need for additional adjusted odds ratios (ORs). The OR of drop-out according to subscales of TDP was not significant for NA (OR=2.45; 95% confidence interval [CI], 0.88–7.09; p=0.09), whereas TDP was as a significant variable for SI (OR=3.07; 95% CI, 1.07–8.80; p<0.05).
A total of 27 participants (42.9%) completed the CR program: 5 were classified as TDP and 22 as non-TDP. In the baseline CPET prior to CR and CPET after CR, the two groups showed no differences in HRpeak, HRrest, SBP, DBP, RPE at stage 3, RPEpeak, VO2peak, and exercise time (Table 5). VO2peak increased from pre-CR to post-CR by 12.6% (26.1 to 29.4 mL/kg/min) in the TDP group and 9.4% (26.7 to 29.2 mL/kg/min) in the non-TDP group. The non-TDP group showed a significant increase in VO2peak from pre-CR to post-CR. However, despite the higher rate of increase in the TDP group, it was not statistically significant as the number of participants in the group was too small. Repeated-measures ANOVA results showed no significant differences in group-period interaction according to TDP, while a linear mixed model adjusted for anxiety, depression, and BMI also showed no significant differences in group-period interaction according to TDP.
Prevalence and mortality rates associated with CVDs remain at a high level, and studies continue to investigate the known CVD risk factors and psychosocial factors [13]. TDP refers to underlying traits in an individual, which do not change easily, unlike emotions. TDP induces negative states, such as depression and anxiety [14], with a continued influence on behavioral patterns, and negative biological responses in the body [4]. International studies have recently associated TDP with CVDs including heart failure, CAD, and hypertension [15,16]. Further relevant studies are required. Previous TDP-related studies have reported a high recurrence of cardiac events [17] and low quality of life [18] among patients diagnosed with coronary artery syndrome, while the negative effects of TDP in heart diseases manifest via specific behavioral patterns and biological pathways [19]. However, scant information is available concerning the direct comparison of effects based on exercise test results obtained from short-term CR and CR participation rates. The present study investigated the CR participation rate, effects on mental health, and effects of CR in CAD patients with TDP.
One-third of the participants were classified as TDP, similar to the previously reported frequency of 24%–37% in studies conducted outside of Korea [20-22]. Studies in Korea reported TDP rates of 27.4% and 38.0% [23,24].
With respect to demographic characteristics, the TDP group displayed lower BMI, which is contrary to the higher BMI reported in TDP subjects in a study from Iceland [25]. In that study, the demographic characteristics of 4,753 people randomly selected from the general population were investigated; and those with TDP showed a higher prevalence of hypertension, diabetes, waist circumference, and BMI. The present study had a smaller sample size and only analyzed CAD patients and not the general population.
In our study, the TDP group was associated with depression and anxiety, consistent with prior studies. A recent meta-analysis of the association between mental distress and TDP revealed a greater than 2-fold risk for mental health problems compared with subjects without TDP [26]. Moreover, various studies have also indicated positive correlations between TDP and anxiety and depression, even after adjusting for disease severity and demographic differences [27-29]. Anxiety and depression are common mental health issues among CVD patients, and increase the incidence of CVD, diminish the quality of life and attitudes to treatment, and increase the prognosis of death [30]. TDP reinforces a negative emotional state and increases the prevalence and mortality rates associated with CVD [31]. Therefore, management of TDP patients via psychological intervention for CVD and treatment of NA conditions should be carried out together with CR. The results of such interventions need further investigation and assessment.
Currently, no interventions or treatments are available to directly target TDP. Consequently, little is known about the practical methods for enhancing the prognosis of TDP patients [19]. Future interventions and treatments should focus on the mechanisms of TDP association with the poor prognosis of heart disease [32,33].
Concerning the negative effect of TDP on heart disease, two potential mechanisms involving behavioral patterns and biological pathways have been reported [34,35]. The behavioral pattern pathway of TDP in terms of specific health-risk behavior corresponds to an indirect mechanism, while the biological pathway represents a direct mechanism associated with direct physiological responses that occur when TDP patients experience chronic stress. Significant associations of TDP with the hypothalamus-pituitary-adrenal axis imbalance [36], hyper-activation of the cardiovascular system [37], increased inflammatory and immune responses [38], and metabolite network dysfunction [39] suggest underlying biological mechanisms.
The present study attempted to identify changes according to TDP in relation to capacity for aerobic exercise based on CR. No difference was found between TDP and non-TDP subjects due to the small sample size. Conversely, the conflicting findings from prior studies may be related to attempts to identify the biological mechanism based on laboratory findings from blood tests, metabolites, and immune responses. Future studies should increase the number of participants and statistical power considering the high drop-out or loss rates.
In the present study, the TDP group showed a significantly lower CR rate of participation than the non-TDP group, while the OR of CR loss according to TDP was 3.87, which is consistent with previous reports suggesting that TDP subjects have poor drug compliance and do not engage in healthy lifestyles [20,40]. The presence of TDP was the only variable associated with the CR participation rate. Depression and anxiety did not show such associations. In this context, among the subscales of TDP, SI but not NA was correlated with the CR participation rate. Based on this finding, it appears that TDP may probably induce NA frequently and promote other behavioral patterns unrelated to NA, which are mostly due to the underlying disposition to induce SI.
TDP patients are more likely to continue or revert to unhealthy at-risk behavior, and abstain from aerobic exercise, dieting, smoking cessation, drug compliance, and behavioral changes [20,29,40]. Because of such risky behaviors, TDP patients show passive responses towards the treatment of heart disease, with negative views on treatment efficacy and procedures. In the present study, TDP patients were monitored for their CR participation rate only, while an overall survey including dietary modification, aerobic exercise, transition from a smoker to a non-smoker, and adherence to prescription medication was not conducted. Future studies should conduct comprehensive surveys of health-risk behaviors in relation to TDP and a systematization of CR programs that includes correction of health risk behaviors and the need for relevant interventions.
CR is not simply a matter of engaging in aerobic training. It also includes abstinence from smoking and alcohol consumption, dietary management, stress reduction, education concerning and prescription of aerobic exercise, and eliminating barriers to exercise. All these measures should be reinforced during regular and scheduled follow-up sessions. Moreover, when a cardiac event is involved, CPET is initially performed within 2–3 months, and every 6–12 months thereafter, which provides feedback for encouraging dynamic participation from the patients. In the present study, differences were found in the CR participation rate and the mental health state according to TDP, but no difference in aerobic exercise capacity post-CR was detected. Therefore, in the long-term, the CR participation rate may decline among the TDP group due to their risky health behavior, resulting in a lack of aerobic exercise capacity, shift to a less healthy lifestyle, and lack of risk factor management. Such outcomes suggest a high recurrence rate of long-term cardiac events and a high mortality rate in the TDP group [35].
The limitations in the present study concern the number of participants lost to follow-up and the higher than anticipated drop-out during CR, which resulted in only a small number of participants completing the CR exercise program to analyze the effects of CR. As a result, statistical power was reduced. Moreover, the survey relating to the health behavior of the participants was not comprehensive, and failed to reflect individual differences in duration, intensity, and frequency of aerobic exercise. In the future, additional systematic studies that address these issues will be required.
In conclusion, CAD patients with TDP showed a low CR participation rate. Among those who completed the CR exercise program, no significant differences were detected in efficacy of CR according to TDP. Participants with TDP showed higher levels of anxiety and depression. Consistent with previous studies reporting the association between TDP and a negative prognosis in heart disease, the present study presents further evidence and identifies a potential behavioral pathway, while also confirming similar findings among the subcategories, with SI playing a key role. Currently, there is a strong interest in Korea for any evidence to increase the CR participation rate. Future studies that consider TDP, when developing and applying psychosocial interventions for increasing CR participation rates are needed. Increasing the CR participation rate of patients with heart disease in actual clinical settings should improve the long-term prognosis.
Supplementary Materials
Supplementary materials can be found via http://doi.org/10.5535/arm.2018.42.5.748.
REFERENCES
1. McCranie EW, Watkins LO, Brandsma JM, Sisson BD. Hostility, coronary heart disease (CHD) incidence, and total mortality: lack of association in a 25-year follow-up study of 478 physicians. J Behav Med. 1986; 9:119–25.

2. Pedersen SS, Denollet J, Ong AT, Serruys PW, Erdman RA, van Domburg RT. Impaired health status in Type D patients following PCI in the drug-eluting stent era. Int J Cardiol. 2007; 114:358–65.

3. Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychol Rev. 1989; 96:234–54.

4. Pedersen SS, Middel B. Increased vital exhaustion among type-D patients with ischemic heart disease. J Psychosom Res. 2001; 51:443–9.

5. Thompson PD, Arena R, Riebe D, Pescatello LS; American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013; 12:215–7.

6. Kim C, Kim YJ. The effects of Karvonen exercise prescription in acute coronary artery disease patients reaching age-predicted maximal heart rates with exercise stress test. J Exp Biomed Sci. 2013; 19:254–60.
7. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate: a longitudinal study. Ann Med Exp Biol Fenn. 1957; 35:307–15.
8. Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001; 104:1694–740.
9. Lee MS, Park YM, Lim HE, Song WH, Ahn JC, Kim SH, et al. Preliminary study on the standardization of Korean version of type D personality scale 14: internal consistency and construct validity. Korean J Psychosom Med. 2007; 15:81–7.
10. Denollet J. DS14: standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosom Med. 2005; 67:89–97.

11. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–70.

12. Oh SM, Min KJ, Park DB. A study on the standardization of the hospital anxiety and depression scale for Koreans: a comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999; 38:289–96.
13. Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. BMJ. 1999; 318:1460–7.

14. Pedersen SS, Holkamp PG, Caliskan K, van Domburg RT, Erdman RA, Balk AH. Type D personality is associated with impaired health-related quality of life 7 years following heart transplantation. J Psychosom Res. 2006; 61:791–5.

15. Aquarius AE, Denollet J, Hamming JF, De Vries J. Role of disease status and type D personality in outcomes in patients with peripheral arterial disease. Am J Cardiol. 2005; 96:996–1001.

16. Pedersen SS, van Domburg RT, Theuns DA, Jordaens L, Erdman RA. Type D personality is associated with increased anxiety and depressive symptoms in patients with an implantable cardioverter defibrillator and their partners. Psychosom Med. 2004; 66:714–9.

17. Denollet J, Pedersen SS, Vrints CJ, Conraads VM. Usefulness of type D personality in predicting five-year cardiac events above and beyond concurrent symptoms of stress in patients with coronary heart disease. Am J Cardiol. 2006; 97:970–3.

18. Sogaro E, Schinina F, Burgisser C, Orso F, Pallante R, Aloi T, et al. Type D personality impairs quality of life, coping and short-term psychological outcome in patients attending an outpatient intensive program of cardiac rehabilitation. Monaldi Arch Chest Dis. 2010; 74:181–91.

19. Cao X, Wong EM, Chow Choi K, Cheng L, Ying Chair S. Interventions for cardiovascular patients with Type D personality: a systematic review. Worldviews Evid Based Nurs. 2016; 13:314–23.

20. Kupper N, Pedersen SS, Hofer S, Saner H, Oldridge N, Denollet J. Cross-cultural analysis of type D (distressed) personality in 6222 patients with ischemic heart disease: a study from the International HeartQoL Project. Int J Cardiol. 2013; 166:327–33.

21. Vukovic O, Tosevski DL, Jasovic-Gasic M, Damjanovic A, Zebic M, Britvic D, et al. Type D personality in patients with coronary artery disease. Psychiatr Danub. 2014; 26:46–51.
22. Yu XN, Chen Z, Zhang J, Liu X. Coping mediates the association between Type D personality and perceived health in Chinese patients with coronary heart disease. Int J Behav Med. 2011; 18:277–84.

23. Park JH, Tahk SJ, Bae SH. Impact of type D personality on health status and health behaviors in patients with coronary artery disease. Korean J Health Promot. 2010; 10:123–30.
24. Son HM. Quality of life and illness intrusiveness by type-D personality in the patients with coronary artery disease. J Korean Acad Nurs. 2009; 39:349–56.

25. Svansdottir E, Denollet J, Thorsson B, Gudnason T, Halldorsdottir S, Gudnason V, et al. Association of type D personality with unhealthy lifestyle, and estimated risk of coronary events in the general Icelandic population. Eur J Prev Cardiol. 2013; 20:322–30.

26. Versteeg H, Spek V, Pedersen SS, Denollet J. Type D personality and health status in cardiovascular disease populations: a meta-analysis of prospective studies. Eur J Prev Cardiol. 2012; 19:1373–80.

27. Hausteiner C, Klupsch D, Emeny R, Baumert J, Ladwig KH; KORA Investigators. Clustering of negative affectivity and social inhibition in the community: prevalence of type D personality as a cardiovascular risk marker. Psychosom Med. 2010; 72:163–71.

28. Starrenburg AH, Kraaier K, Pedersen SS, van Hout M, Scholten M, van der Palen J. Association of psychiatric history and type D personality with symptoms of anxiety, depression, and health status prior to ICD implantation. Int J Behav Med. 2013; 20:425–33.

29. Svansdottir E, van den Broek KC, Karlsson HD, Gudnason T, Denollet J. Type D personality is associated with impaired psychological status and unhealthy lifestyle in Icelandic cardiac patients: a cross-sectional study. BMC Public Health. 2012; 12:42.

30. Pedersen SS, Denollet J, van Gestel YR, Serruys PW, van Domburg RT. Clustering of psychosocial risk factors enhances the risk of depressive symptoms 12-months post percutaneous coronary intervention. Eur J Cardiovasc Prev Rehabil. 2008; 15:203–9.

31. Williams L, O’Connor RC, Howard S, Hughes BM, Johnston DW, Hay JL, et al. Type-D personality mechanisms of effect: the role of health-related behavior and social support. J Psychosom Res. 2008; 64:63–9.

32. Pedersen SS, Schiffer AA. The distressed (Type D) personality: a risk marker for poor health outcomes in ICD patients. Herzschrittmacherther Elektrophysiol. 2011; 22:181–8.
33. Pelle AJ, Erdman RA, van Domburg RT, Spiering M, Kazemier M, Pedersen SS. Type D patients report poorer health status prior to and after cardiac rehabilitation compared to non-type D patients. Ann Behav Med. 2008; 36:167–75.

34. Denollet J, Schiffer AA, Spek V. A general propensity to psychological distress affects cardiovascular outcomes: evidence from research on the type D (distressed) personality profile. Circ Cardiovasc Qual Outcomes. 2010; 3:546–57.
35. Pedersen SS, Denollet J. Is Type D personality here to stay? Emerging evidence across cardiovascular disease patient groups. Curr Cardiol Rev. 2006; 2:205–13.

36. Whitehead DL, Perkins-Porras L, Strike PC, Magid K, Steptoe A. Cortisol awakening response is elevated in acute coronary syndrome patients with type-D personality. J Psychosom Res. 2007; 62:419–25.

37. Habra ME, Linden W, Anderson JC, Weinberg J. Type D personality is related to cardiovascular and neuroendocrine reactivity to acute stress. J Psychosom Res. 2003; 55:235–45.

38. Mommersteeg PM, Pelle AJ, Ramakers C, Szabo BM, Denollet J, Kupper N. Type D personality and course of health status over 18 months in outpatients with heart failure: multiple mediating inflammatory biomarkers. Brain Behav Immun. 2012; 26:301–10.

Fig. 1.
Flow diagram outlining progress of subjects in the study. CR, cardiac rehabilitation; CPET, cardiopulmonary exercise test.
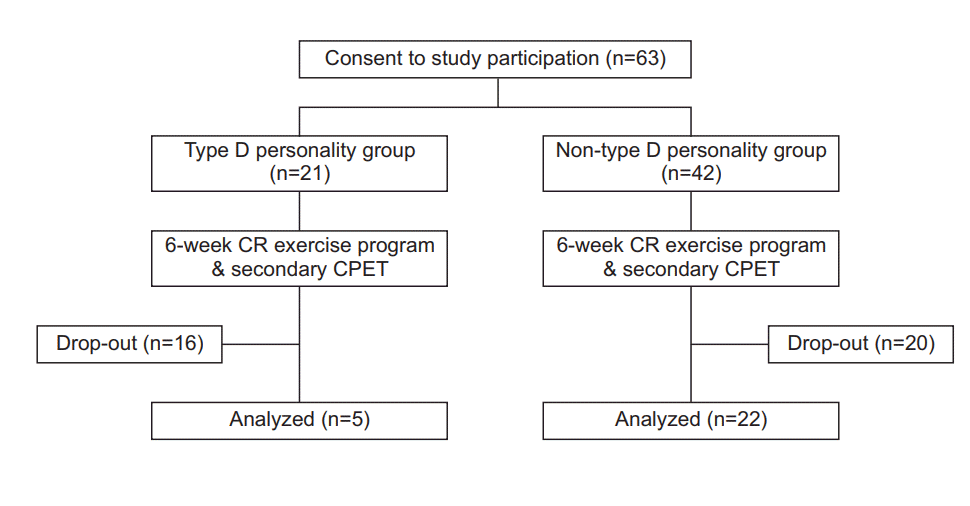
Table 1.
Classification of type D and non-type D personalities using TDP scale
| Total | Type D (n=21) | Non-type D (n=42) | p-value | |
|---|---|---|---|---|
| Negative affectivity | 9.3±5.4 | 14.0±3.7 | 6.9±4.5 | <0.001* |
| Social inhibition | 9.1±5.2 | 14.5±3.5 | 6.3±3.6 | <0.001* |
Table 2.
Participants’ baseline demographic characteristics: type D vs. non-type D personality
| Characteristic | Type D (n=21) | Non-type D (n=42) | p-value |
|---|---|---|---|
| Age (yr) | 62.2±11.6 | 58.6±10.8 | 0.218 |
| Sex | 0.698 | ||
| Male | 14 | 30 | |
| Female | 7 | 12 | |
| BMI (kg/m2) | 24.6±2.5 | 26.1±2.7 | 0.038* |
| LVEF (%) | 49.6±13.3 | 52.6±13.6 | 0.409 |
| Smoking | |||
| Non-smoker | 6 (28.6) | 17 (40.5) | 0.205 |
| Current smoker | 8 (38.1) | 19 (45.2) | 0.205 |
| Ex-smoker | 7 (33.3) | 6 (14.3) | 0.205 |
| Diagnosis | |||
| UA | 7 (33.3) | 8 (19.0) | 0.209 |
| AMI | 14 (66.7) | 34 (81.0) | 0.209 |
| DM | 10 (47.6) | 13 (31.0) | 0.195 |
| Hypertension | 13 (61.9) | 26 (61.9) | 1 |
| No. of diseased vessel | 0.81 | ||
| One | 10 (47.6) | 27 (64.3) | |
| Two | 7 (33.3) | 13 (31.0) | |
| Three | 4 (19.0) | 2 (4.8) | |
| Dyslipidemia | 5 (31.3) | 11 (26.2) | 0.838 |
Table 3.
Differences in anxiety and depression in type-D personality
| Type D (n=21) | Non-type D (n=42) | p-value | |
|---|---|---|---|
| Depression | 12 (57.1) | 13 (31.0) | 0.045* |
| Anxiety | 8 (38.1) | 4 (9.5) | 0.014* |
Table 4.
Results of logistic regression analysis predicting the CR drop-out rate, depression, and anxiety in type-D personality
| B | SE | Wald | Diff. | p-value | OR 95% CI | |
|---|---|---|---|---|---|---|
| CR drop-out rate | 1.354 | 0.60 | 5.11 | 1 | 0.024 | 3.87 (1.20–12.53) |
| Depression | 1.090 | 0.55 | 3.89 | 1 | 0.049 | 2.97 (1.01–8.79) |
| Anxiety | 1.766 | 0.69 | 6.52 | 1 | 0.011 | 5.85 (1.51–22.67) |
Table 5.
Exercise parameters pre-CR vs. post-CR
| Time | Type D group (n=5) | Non-type D group (n=22) | |
|---|---|---|---|
| HRrest (beats/min) | Baseline | 68.6±10.8 | 68.2±11.9 |
| 6 weeks | 68.0±11.7 | 63.1±10.5 | |
| HRpeak (beats/min) | Baseline | 130.6±21.7 | 138.4±18.9 |
| 6 weeks | 138.4±24.2 | 137.5±20.8 | |
| SBPrest (mmHg) | Baseline | 116.4±15.3 | 120.1±16.9 |
| 6 weeks | 120.6±11.3 | 122.3±15.6 | |
| DBPrest (mmHg) | Baseline | 83.8±7.6 | 81.7±7.8 |
| 6 weeks | 78.4±6.5 | 82.0±7.7 | |
| SBPpeak (mmHg) | Baseline | 163.6±38.2 | 162.6±27.3 |
| 6 weeks | 174.2±25.2 | 176.5±23.2* | |
| DBPpeak (mmHg) | Baseline | 83.6±18.6 | 83.1±12.3 |
| 6 weeks | 88.6±11.9 | 86.2±13.0 | |
| ETT time (s) | Baseline | 815.0±307.6 | 853.1±186.1 |
| 6 weeks | 937.0±140.5 | 936.3±169.4* | |
| VO2peak (mL/kg/min) | Baseline | 26.1±12.0 | 26.7±7.9 |
| 6 weeks | 29.4±13.1 | 29.2±7.9* | |
| ΔVO2peak (%) | Baseline | - | - |
| 6 weeks | 12.64 | 9.36 | |
| RPP at stage 3 | Baseline | 11,988±2,490 | 14,881±4,868 |
| 6 weeks | 13,497±2,725 | 13,464±2,563 | |
| RPE at stage 3 | Baseline | 9.8±2.2 | 10.4±2.6 |
| 6 weeks | 10.2±2.8 | 9.4±1.9 | |
| RERpeak | Baseline | 1.13±0.17 | 1.10±0.10 |
| 6 weeks | 1.12±0.18 | 1.14±0.10 |
Values are presented as mean±standard deviation.
CR, cardiac rehabilitation; HRrest, resting heart rate; HRpeak, peak heart rate; SBPrest, resting systolic blood pressure; DBPrest, resting diastolic blood pressure; SBPpeak, peak systolic blood pressure; DBPpeak, peak diastolic blood pressure; ETT, exercise tolerance test; VO2peak, peak oxygen uptake; ΔVO2peak, improvement rate of VO2peak; RPP, rate pressure product; RPE, rated perceived exertion; RERpeak, peak respiratory exchange ratio.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download