Abstract
Background
Presepsin has emerged as a prognostic marker for both coronavirus disease-2019 and acute kidney injury. To assess the feasibility of requesting presepsin tests using Sysmex HISCL-5000 (Sysmex, Japan), we evaluated sample stability under various conditions.
Methods
We utilized leftover samples previously analyzed for presepsin using the PATHFAST immune analyzer (LSI Medience Corporation, Japan) to evaluate the stability across four scenarios: five-day cold storage, mixing on a complete blood count (CBC) platform, whole blood transfer, and correlation between HISCL presepsin and PATHFAST tests. A total of 102 specimens were analyzed.
Results
Refrigerated serum samples exhibited progressive increases in presepsin levels over five days, with significant rises of 3.6% at six hours, 8.05% at two days, and 14.25% at five days (P=0.016). Conversely, plasma maintained stable levels throughout the five-day cold storage period (P=0.14). Compared to manual invert mixing, CBC platform mixing significantly elevated presepsin values (P<0.001). Notably transferring whole blood followed by centrifugation within 12 hours yielded results comparable to those obtained from plasma. The correlation between HISCL and PATHFAST tests was excellent, with a Pearson correlated coefficient of r=0.9844.
Conclusions
Based on our findings, serum samples are unsuitable for laboratory referral of presepsin due to their instability in refrigeration. EDTA plasma, however, exhibits excellent stability for five days under refrigerated conditions. Alternatively, transferring whole blood followed by centrifugation within 12 hours presents a viable option for presepsin testing.
초록
배경
Presepsin은 코로나바이러스 질병 2019 (COVID-19) 및 급성 신장 손상에 대한 예후 표지자로 사용될 수 있다. 이 연구에서는 시료의 안정성을 여러 조건에서 평가하여 Sysmex HISCL-5000 (Sysmex, Japan) presepsin 검사를 수탁검사로 실시하기에 적합한지 확인하였다.
방법
PATHFAST 면역분석기(LSI Medience Corporation, Japan)를 이용한 presepsin 검사 후 남은 검체에 대한 시료 안정성을 평가하였다. 총 102개의 검체를 사용하여 5일간의 냉장 보관, CBC 플랫폼을 이용한 혼합 및 전혈의 이송 중 안정성을 평가했다. HISCL presepsin과 PATHFAST presepsin 테스트 간의 상관관계는 39개 검체에 대해 평가했다.
Sepsis, a life-threatening organ dysfunction triggered by a dysregulated host response to infection [1], remains a global challenge with significant morbidity and mortality [2]. Early detection and prompt treatment of high-risk septic patients are crucial for improving outcomes [3].
Presepsin, a fragment of the CD14 protein identified as a soluble subtype, emerges as a promising biomarker for sepsis diagnosis and prognosis [4-7]. Secreted by lipopolysaccharide-recognizing activated monocytes, presepsin offers advantages over existing markers like procalcitonin and C-reactive protein, reportedly releasing earlier and reaching peak concentrations faster [4, 8, 9]. Presepsin’s potential as a prognostic marker has recently gained traction, particularly for coronavirus disease-2019 (COVID-19) patients. Studies have demonstrated its ability to enhance outcome prediction when combined with clinical indices, exceeding the accuracy of clinical indices alone [10]. Presepsin levels in moderate COVID-19 cases were significantly higher than in mild cases, suggesting its value in guiding treatment and prognosis decisions [11]. Moreover, presepsin levels have shown promise in predicting septic acute kidney injury [12].
Rapid turnaround times are crucial for sepsis diagnostic biomarkers. However, situations arise where limited laboratory resources necessitate test transfer to reference laboratories. In such cases, the sample stability during transport for Sysmex HISCL-5000 presepsin testing becomes a critical factor. This study aims to evaluate the stability of samples under such transport conditions.
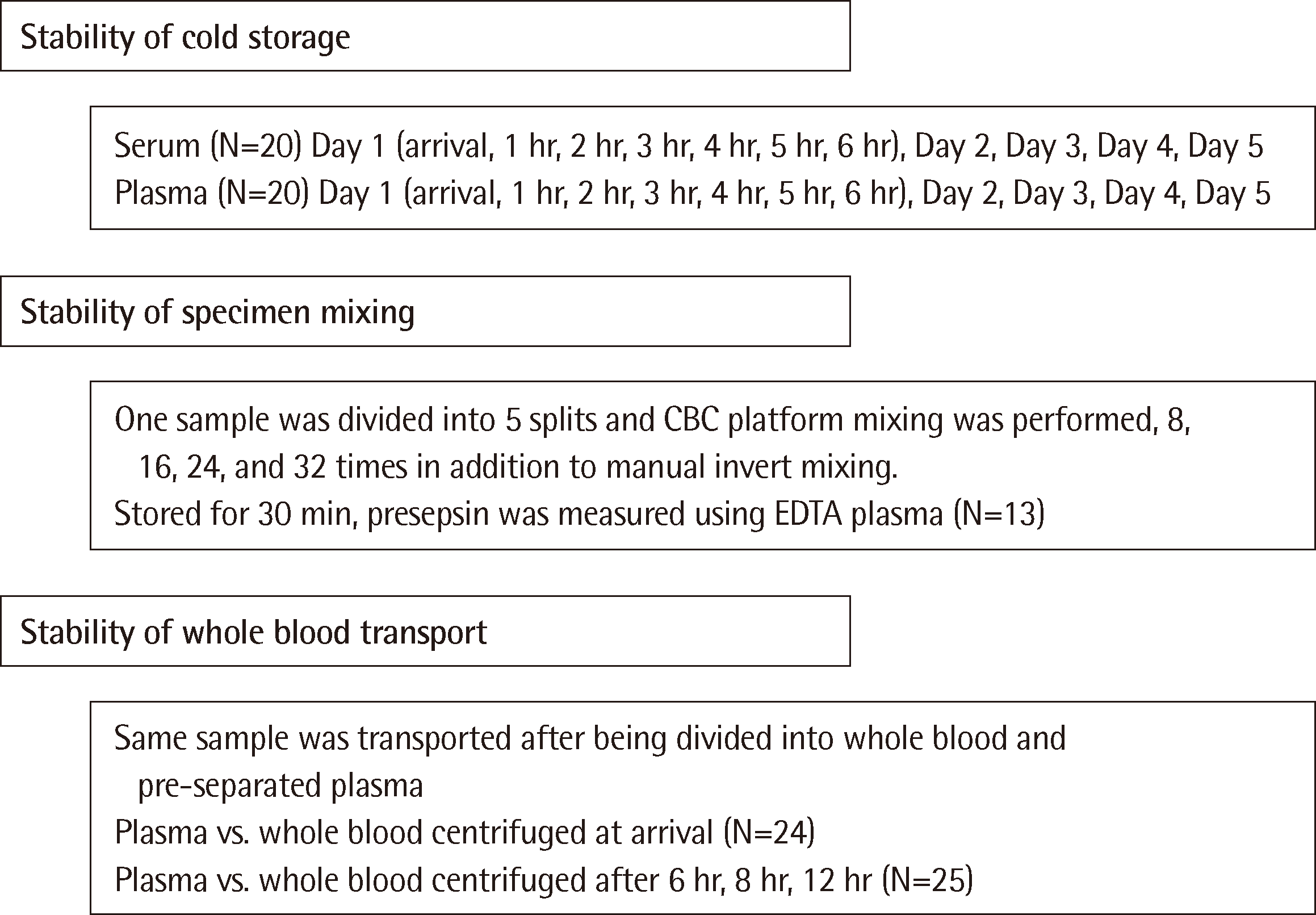
This study utilized leftover samples previously analyzed for presepsin using a PATHFAST immune analyzer (LSI Medience Corporation, Japan). These samples, originally requested from June to July 2022, were employed to evaluate presepsin stability across 102 specimens transferred to the Seegene Medical Foundation Busan Gyeongnam Laboratory Center. Fig. 1 provides an overview of the specimen stability assessment.
In the Seegene laboratory, presepsin levels were measured using the HISCL Presepsin assay (Sysmex, Kobe, Japan) on an HISCL 5000 automated analyzer (Sysmex). This analyzer employs a delayed one-step sandwich chemiluminescence enzyme immunoassay. The manufacturer-specified reference range for HISCL presepsin is up to 333.0 pg/mL. Both PATHFAST and HISCL presepsin assays were performed strictly according to their respective manufacturer’s instructions. The Institutional Review Board of Dong-A University Hospital approved this study (IRB No. 21-246).
To assess sample stability during cold storage, we transported 25 serum and 20 EDTA plasma samples from Dong-A University Hospital to the Seegene laboratory under refrigerated conditions. HISCL presepsin levels were measured immediately upon arrival and repeated hourly for the first six hours. Subsequent measures were taken daily for five days.
The stability of presepsin levels under different mixing conditions was evaluated using 13 whole blood samples. Following manual invert mixing, the samples were subjected to 8, 16, 24, and 32 additional mixings on the inversion mode of a CBC platform mixer (XN-2000, Sysmex Hematology Analyzer, Kobe, Japan). After a 30-minute resting period, the whole blood was centrifuged and HISCL presepsin levels were measured in the resulting EDTA plasma.
To assess the stability of presepsin during whole blood transfer, we divided 24 EDTA whole blood specimens from Dong-A University Hospital laboratory into two aliquots. One aliquot was sent directly as whole blood, while the other was centrifuged and the resulting EDTA plasma was sent to the reference laboratory. Upon arrival, presepsin levels were measured simultaneously in both the EDTA plasma from the centrifuged whole blood, and directly transferred whole blood in 24 samples. Additionally, 25 specimens sent as whole blood were centrifuged after 6, 8, and 12 hours (5, 10, and 10 specimens, respectively) under refrigerated conditions. Presepsin concentration was then measured in the centrifuged plasma and compared to the initial levels measured in the directly transferred EDTA plasma from the same specimens.
To evaluate the correlation between the PATHFAST and HISCL presepsin assays, we used leftover EDTA plasma with 39 clinical samples after PATHFAST measurement. These aliquots were divided into two, frozen at -80℃ for two weeks, and then thawed. Presepsin levels were subsequently measured in both samples from each aliquot, once with the PATHFAST and once with the HISCL assay.
Presepsin stability throughout five-day cold storage was evaluated using repeated measures analysis of variance (ANOVA) and linear trend analysis for both serum and EDTA plasma. Similarly, repeated measures ANOVA assessed changes in presepsin levels due to CBC platform mixing. To compare presepsin levels in plasma versus whole blood after transfer, Bland-Altman plots were generated. The correlation between PATHFAST and HISCL presepsin measurements was determined by both Passing and Bablok regression and Spearman’s rank correlation. All statistical analyses were performed using MedCalc Software (version 18.11.6, Med- Calc Software, Ostend, Belgium), with a significance level set at P<0.05.
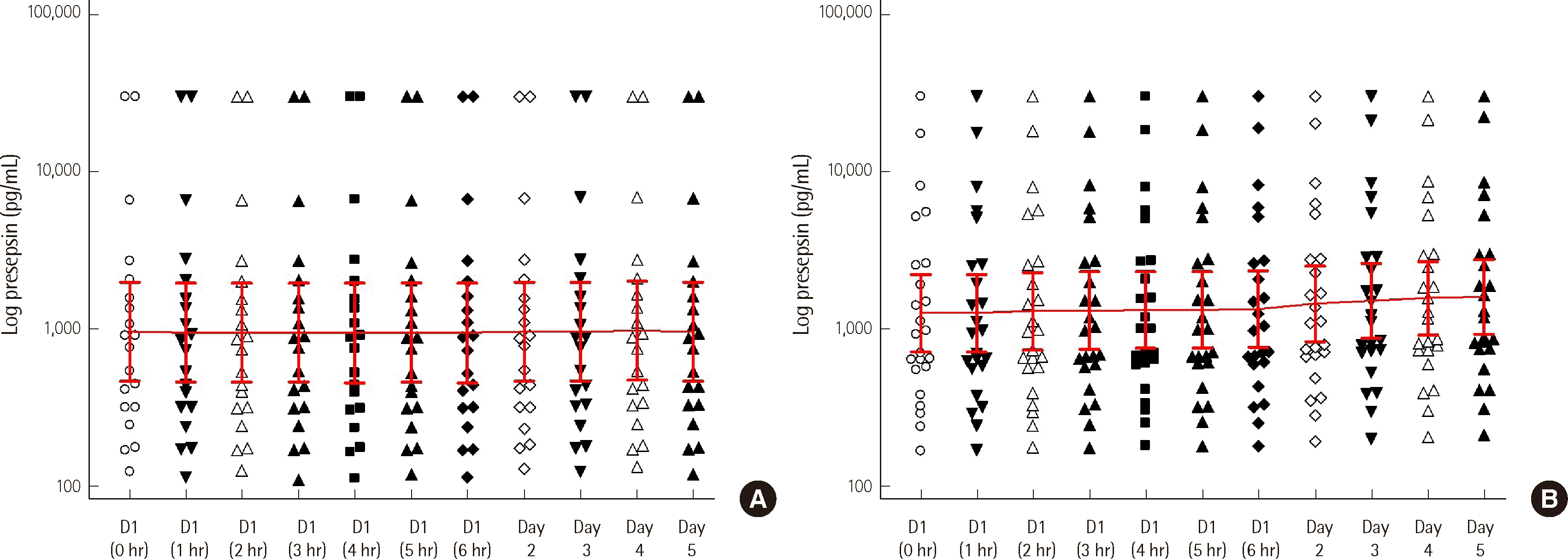
Serum samples (N=25) showed a mean HISCL presepsin level of 3,518.2 pg/mL upon arrival at the Seegene laboratory. This level increased by 3.6% to 3,644.9 pg/mL after six hours of cold storage. Further increases were observed at 8.05% on day 2 (3,801.6 pg/ mL) and 14.25% on day 5 (4,019.6 pg/mL). Linear trend analysis confirmed a statistically significant rise in presepsin levels over time (P=0.016). In contrast, EDTA plasma (N=20) exhibited no significant change in presepsin levels throughout the five-day cold storage period. The mean value upon arrival was 4,039.2 pg/ mL, and it remained essentially unchanged at 4,043.2 pg/mL on day 5 (P=0.14) (Table 1) (Fig. 2).
Compared to manual invert mixing, CBC platform mixing led to a significant increase in mean presepsin level for all mixing times evaluated (8 times to 32 times, P<0.001). The highest mean level was observed at 16 times mixing, at 106.3 pg/mL (95% confidence interval [CI] 87.6–125.0 pg/mL) (Fig. 3).
To assess the impact of pre-centrifugation on whole blood transfer stability, a Bland-Altman plot was generated for presepsin levels in 24 samples. All samples fell within ±1.96 SD of the bias, indicating good agreement between plasma and immediately centrifuged whole blood (Fig. 4). For whole blood stored for 6, 8, and 12 hours before centrifugation, presepsin concentrations were compared to those from directly transferred plasma. All samples but one at 8 and 12 hours, respectively, remained within ±1.96 SD, suggesting acceptable stability for up to 12 hours (Fig. 4).
We evaluated the correlation between PATHFAST and HISCL presepsin values using 39 plasma samples across a range of concentrations. Linearity was well maintained, with no significant deviation detected (correlation coefficient r=0.9844, 95% CI 0.9702– 0.9919, P=0.28) (Fig. 5). Additionally, the Spearman rank correlation coefficient between the two methods was 0.9526, further confirming a strong relationship.
Presepsin’s potential extends beyond sepsis diagnosis, demonstrating promise as a prognostic marker in diverse patient populations. For example, a study of 343 patients undergoing transcatheter aortic valve implantation found a high baseline presepsin level to be an independent predictor of one-year mortality, with an odds ratio of 2.2 [13]. Similarly, increased presepsin concentrations were reported to be associated with 30-day in-hospital mortality and disease severity in 211 emergency department patients with community-acquired pneumonia [14]. Moreover, a prospective cohort study of 108 gastric cancer patients revealed that presepsin levels on postoperative days 3, 5, and 7 served as more reliable predictors of postoperative infectious complications to C-reactive protein, white blood cell, and neutrophil levels [15].
Limited laboratory resources in healthcare facilities often require specimen transfer to referral laboratories. This study, to the best of our knowledge, is the first study to specifically evaluate sample stability for HISCL presepsin testing during transfer. While EDTA plasma remained stable for up to five days, serum tended to increase after 24 hours. This finding is somewhat divergent from the HISCL presepsin package insert, which indicates plasma stability for up to two days and serum instability exceeding 10% after four hours of refrigeration. Our observation 8% increase in serum presepsin at 24 hours suggests potential influence from factors such as cellular hemolysis or enzymatic interactions, like cathepsin D. This difference highlights the unsuitability of serum as a preferred sample for referral testing [6]. We chose to evaluate EDTA plasma due to its prevalence in laboratories for CBC tests and other analyses, but heparin can also be considered as an alternative anticoagulant for presepsin measurement.
To assess the impact of mixing on presepsin concentration, samples were mixed using the built-in mixing platform of the CBC analyzer. Compared to manual invert mixing, CBC platform mixing significantly increased presepsin levels, with the highest mean value observed at 16 mixing cycles. However, after this initial rise, no further increase in presepsin concentration was observed. This finding aligns with a previous study that examined NT-proBNP and presepsin changes in whole blood under different mixing methods [16]. While NT-proBNP levels remained unaffected, whole blood presepsin measured with the PATHFAST device significantly increased with CBC platform mixing compared to other methods. Similarly, our study, measuring presepsin in EDTA plasma after 30 minutes of centrifugation, also observed elevated levels following CBC platform mixing. Therefore, in a clinical setting where CBC is performed first using EDTA whole blood, followed by centrifugation for presepsin measurement in plasma, an increase in presepsin level is expected. Consequently, it is recommended to use separate specimens for CBC and presepsin testing to ensure accurate results.
Transporting whole blood under cold storage, followed by centrifugation at the referral laboratory, emerged as a viable alternative to transporting EDTA plasma for HISCL presepsin testing. This approach has the potential to significantly reduce the workload for healthcare providers, especially in smaller clinics, without compromising test accuracy. Our study demonstrated that whole blood transported within 12 hours yielded test results comparable to those obtained from EDTA plasma, suggesting the feasibility of ordering HISCL presepsin as a whole blood test even in a setting lacking immediate centrifugation capabilities.
Furthermore, we compared the presepsin values measured by HISCL and PATHFAST, two widely used assays, using 39 specimens. The excellent correlation coefficient of 0.9844 (similar to the 0.979 reported previously [17]) and the strong Spearman rank correlation of 0.9525 confirm a high degree of agreement between the two methods. This finding strengthens the case of centralized HISCL presepsin testing, enabling consistent and reliable results compared to point-of-care devices.
This study has some limitations. We primarily focused on evaluating specimen stability and comparing results across different test methods. As a result, we did not delve into specific diseases, measure other sepsis biomarkers, or perform specific bacterial identifications. Further research is warranted to elucidate the clinical utility of these biomarker combinations.
In summary, our findings offer valuable insights into presepsin stability for referral testing. EDTA plasma exhibited stability for up to five days under refrigeration, making it a suitable candidate for transport. Conversely, serum showed elevated presepsin levels at 24 hours, rendering it unsuitable for referral purposes. Notably, when transferred as EDTA whole blood, plasma remained stable for up to 12 hours following centrifugation. However, given the significant increase in presepsin observed with CBC platform mixing, we recommend separate specimen collection for presepsin testing to ensure accurate results.
REFERENCES
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. 2016; The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 315:801–10. DOI: 10.1001/jama.2016.0287. PMID: 26903338. PMCID: PMC4968574.

2. Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. 2020; Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and metaanalysis. Crit Care. 24:239. DOI: 10.1186/s13054-020-02950-2. PMID: 32430052. PMCID: PMC7236499. PMID: 78066dc5fc684a03a9f07b028c5daf9d.

3. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. 2021; Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47:1181–247. DOI: 10.1007/s00134-021-06506-y. PMID: 34599691. PMCID: PMC8486643.
4. Yaegashi Y, Shirakawa K, Sato N, Suzuki Y, Kojika M, Imai S, et al. 2005; Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother. 11:234–8. DOI: 10.1007/s10156-005-0400-4. PMID: 16258819.

5. Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. 2015; Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 450:97–103. DOI: 10.1016/j.cca.2015.06.026. PMID: 26164388.

6. Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. 2011; Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 17:764–9. DOI: 10.1007/s10156-011-0254-x. PMID: 21560033.

7. Poggi C, Lucenteforte E, Petri D, De Masi S, Dani C. 2022; Presepsin for the diagnosis of neonatal early-onset sepsis: a systematic review and metaanalysis. JAMA Pediatr. 176:750–8. DOI: 10.1001/jamapediatrics.2022.1647. PMID: 35639395. PMCID: PMC9157383.

8. Kahveci U, Ozkan S, Melekoglu A, Usul E, Ozturk G, Cetin E, et al. 2021; The role of plasma presepsin levels in determining the incidence of septic shock and mortality in patients with sepsis. J Infect Dev Ctries. 15:123–30. DOI: 10.3855/jidc.12963. PMID: 33571154.

9. Masson S, Caironi P, Spanuth E, Thomae R, Panigada M, Sangiorgi G, et al. 2014; Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: data from the Albumin Italian Outcome Sepsis trial. Crit Care. 18:R6. DOI: 10.1186/cc13183. PMID: 24393424. PMCID: PMC4056046.

10. Park M, Hur M, Kim H, Lee CH, Lee JH, Kim HW, et al. 2022; Prognostic utility of procalcitonin, presepsin, and the VACO index for predicting 30-day mortality in hospitalized COVID-19 patients. Ann Lab Med. 42:406–14. DOI: 10.3343/alm.2022.42.4.406. PMID: 35177561. PMCID: PMC8859553.

11. Karacaer C, Sert H, Demirci T, Varım C, Kaya G, Genc AB, et al. 2022; The significance of a novel inflammatory biomarker, presepsin, in predicting disease prognosis in patients with COVID-19. Eur Rev Med Pharmacol Sci. 26:8612–9. DOI: 10.26355/eurrev_202211_30398. PMID: 36459042.
12. Shimoyama Y, Umegaki O, Kadono N, Minami T. 2021; Presepsin values predict septic acute kidney injury, acute respiratory distress syndrome, disseminated intravascular coagulation, and shock. Shock. 55:501–6. DOI: 10.1097/SHK.0000000000001664. PMID: 32925599.

13. Weferling M, Fischer-Rasokat U, Vietheer J, Renker M, Rolf A, Keller T, et al. 2022; Presepsin predicts 1-year all-cause mortality better than N-terminal pro-B-type natriuretic peptide in patients undergoing transcatheter aortic valve implantation. Biomark Med. 16:1209–18. DOI: 10.2217/bmm-2022-0533. PMID: 36861450.

14. Lee KR, Hong DY, Paik JH, Jung HM. 2022; Prognostic value of plasma presepsin and pneumonia severity index in patients with community-acquired pneumonia in the emergency department. Medicina (Kaunas). 58:1504. DOI: 10.3390/medicina58111504. PMID: 36363461. PMCID: PMC9692405. PMID: 1657db8ecd084bcb9dd6cb72721293da.

15. Imai Y, Tanaka R, Honda K, Matsuo K, Taniguchi K, Asakuma M, et al. 2022; The usefulness of presepsin in the diagnosis of postoperative infectious complications after gastrectomy for gastric cancer: a prospective cohort study. Sci Rep. 12:21289. DOI: 10.1038/s41598-022-24780-8. PMID: 36494434. PMCID: PMC9734175. PMID: a80686fa75ac4414b043a66bbbf3d4d2.

16. Ham JY, Song KE. 2016; Impact of specimen mixing methods on presepsin point-of-care test results using whole blood. Clin Chem Lab Med. 54:e151–4. DOI: 10.1515/cclm-2015-0759. PMID: 26485750.

17. Kang T, Yoo J, Choi H, Lee S, Jekarl DW, Kim Y. 2022; Performance evaluation of presepsin using a Sysmex HISCL-5000 analyzer and determination of reference interval. J Clin Lab Anal. 36:e24618. DOI: 10.1002/jcla.24618. PMID: 35870180. PMCID: PMC9459287.

Fig. 3
Changes in HISCL presepsin level with CBC platform mixing (N=13). Specimens were mixed 8 to 32 times using the built-in mixing platform of the CBC analyzer. Bars represent mean values with 95% confidence intervals determined by repeated-measures ANOVA (P<0.001).
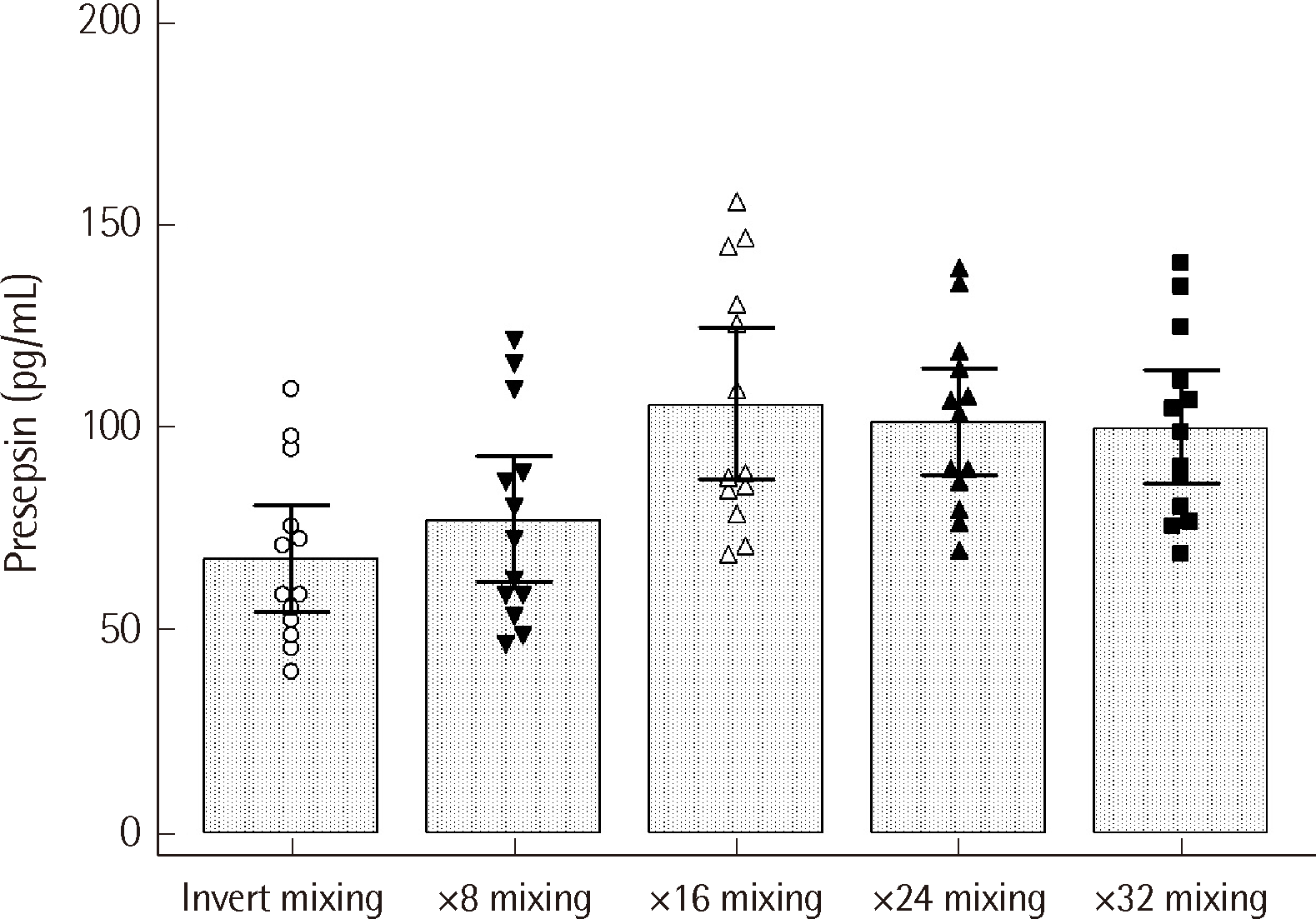
Fig. 4
Stability of whole blood transport. A Bland-Altman plot comparing pre-separated plasma and whole blood centrifuged at different time points. (A) Pre-separated plasma, centrifuged upon arrival (N=24). (B) Whole blood, centrifuged six hours after transfer (N=5). (C) Whole blood, centrifuged eight hours after transfer (N=10). (D) Whole blood, centrifuged 12 hours after transfer (N=10).
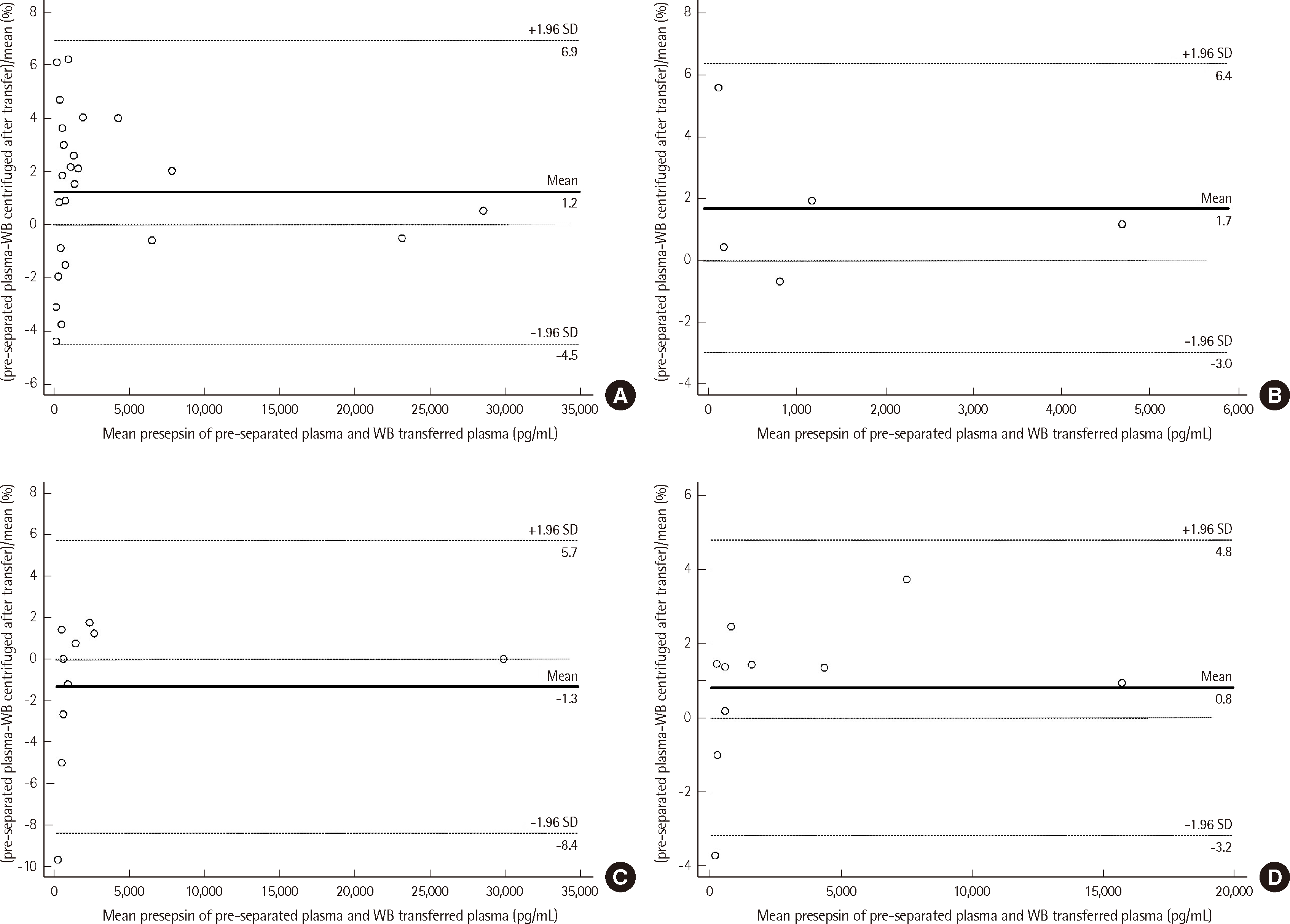
Fig. 5
Correlation between PATHFAST and HISCL presepsin (r=0.9844). The detection limit of PATHFAST presepsin is 20,000 pg/mL.
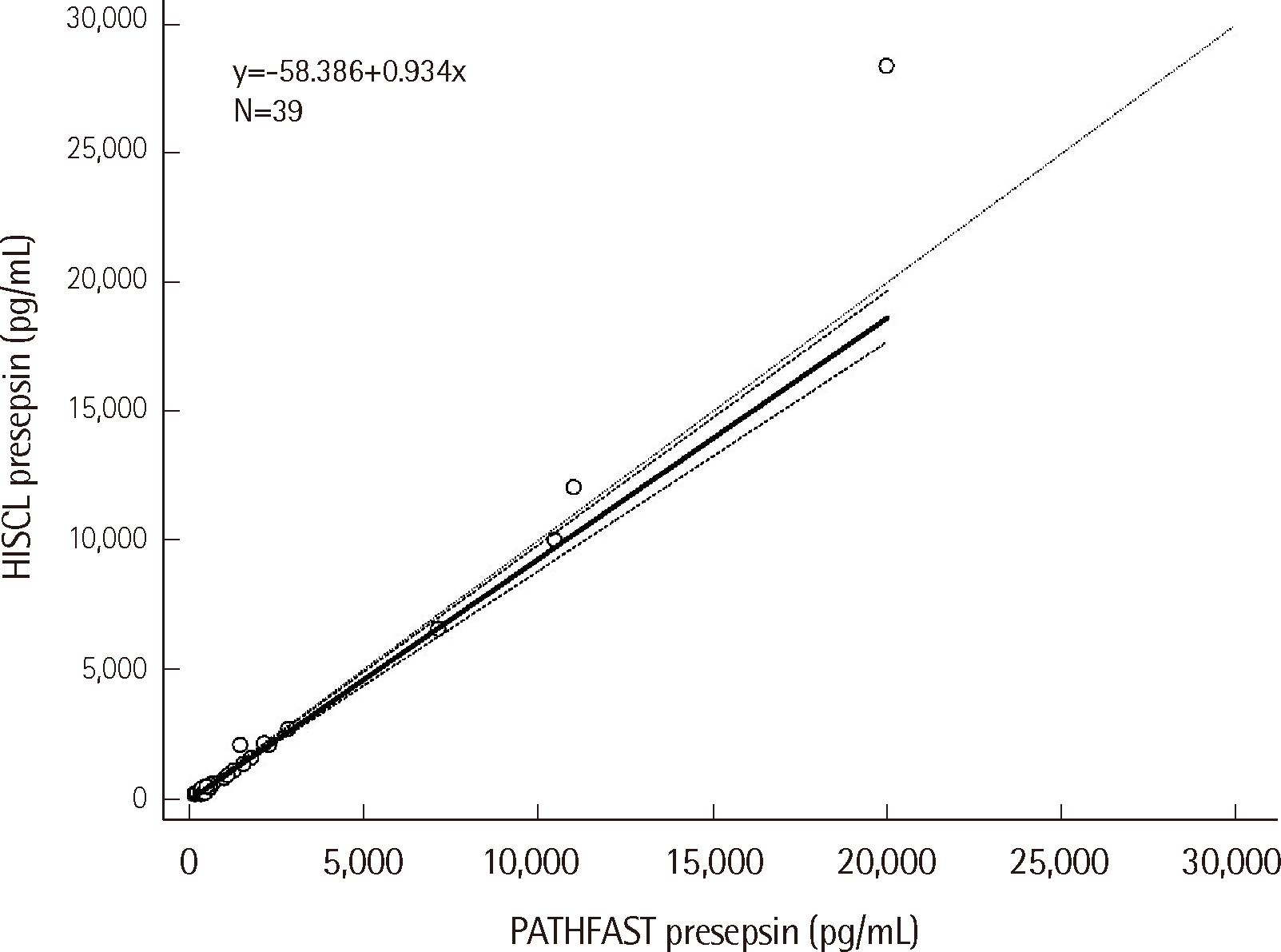
Table 1
Stability of HISCL presepsin in plasma vs. serum for five days
| Mean (pg/mL) | Standard error | 95% Confidence interval | Median (IQR) | |
|---|---|---|---|---|
| Plasma* (N=20) | ||||
| At arrival | 4,039.2 | 2,011.8 | -171.7–8,250.1 | 842.0 (319.7–1,696.7) |
| 1 hr | 4,034.4 | 2,012.3 | -177.4–8,246.3 | 797.5 (321.0–1,676.5) |
| 2 hr | 4,029.7 | 2,012.5 | -182.5–8,241.9 | 808.0 (318.2–1,677.5) |
| 3 hr | 4,028.7 | 2,011.8 | -181.9–8,239.4 | 825.0 (317.7–1,691.0) |
| 4 hr | 4,034.7 | 2,012.7 | -177.9–8,247.4 | 819.0 (311.0–1,674.5) |
| 5 hr | 4,029.0 | 2,012.5 | -183.3–8,241.4 | 812.0 (316.0–1,721.7) |
| 6 hr | 4,036.8 | 2,012.8 | -176.0–8,249.7 | 810.0 (319.0–1,713.5) |
| 2 days | 4,048.9 | 2,012.3 | -162.9–8,260.7 | 831.5 (319.0–1,704.0) |
| 3 days | 4,050.0 | 2,012.7 | -162.6–8,262.7 | 824.5 (331.0–1,719.2) |
| 4 days | 4,064.4 | 2,011.9 | -146.5–8,275.3 | 824.0 (338.7–1,784.0) |
| 5 days | 4,043.2 | 2,012.6 | -169.2–8,255.6 | 810.5 (332.0–1,725.7) |
| Serum† (N=25) | ||||
| At arrival | 3,518.2 | 1,384.6 | 653.8–6,382.7 | 950.5 (566.7–2,565.2) |
| 1 hr | 3,518.1 | 1,388.2 | 646.3–6,389.8 | 960.0 (575.5–2,513.7) |
| 2 hr | 3,578.4 | 1,398.5 | 685.3–6,471.6 | 982.5 (574.2–2,589.0) |
| 3 hr | 3,584.5 | 1,392.7 | 703.4–6,465.6 | 1,003.5 (588.5–2,662.5) |
| 4 hr | 3,597.7 | 1,399.3 | 702.8–6,492.6 | 1,003.5 (602.5–2,711.0) |
| 5 hr | 3,612.5 | 1,401.3 | 713.5–6,511.4 | 1,001.0 (611.5–2,645.2) |
| 6 hr | 3,644.9 | 1,412.4 | 723.0–6,566.8 | 1,011.5 (610.0–2,625.2) |
| 2 days | 3,801.6 | 1,438.1 | 826.7–6,776.6 | 1,103.5 (680.5–2,761.0) |
| 3 days | 3,900.0 | 1,451.8 | 896.7–6,903.3 | 1,158.0 (722.2–2,847.2) |
| 4 days | 3,965.3 | 1,460.5 | 943.9–6,986.8 | 1,222.0 (729.7–2,937.7) |
| 5 days | 4,019.6 | 1,477.7 | 962.7–7,076.5 | 1,240.5 (749.5–2,948.2) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download