Dear Editor,
Mucormycosis is a rare but life-threatening infection in immunocompromised patients. Lichtheimia is the third most-frequently isolated mucoralean genus and is responsible for 5.3% and 19% of infections in the USA and Europe, respectively [1, 2]. It is common in patients with hematologic malignancies and associated with a high mortality (56%). Only three out of the five species within the genus cause mucormycosis: Lichtheimia corymbifera, L. ramosa, and L. ornata. L. corymbifera and L. ornata are not easily distinguishable by their colony or spore/sporangiophore morphology, although they have comparable virulence [3]. Recent reports from India have highlighted that Lichtheimia tends to be invasive, which may result in fatal outcomes, particularly in patients who are immunocompromised or with COVID-19 [4, 5]. Here, we describe a case of pulmonary mucormycosis caused by L. ornata. To the best of our knowledge, this is the first Korean case of pulmonary L. ornata infection. The Institutional Review Board of the Catholic University of Korea, Seoul, Korea, approved the study (KC23ZASI0172) and waived the requirement for informed consent.
In December 2022, a 67-yr-old patient with acute lymphocytic leukemia was admitted to Seoul St. Mary’s Hospital for induction chemotherapy. Chemotherapy was initiated on day 3. Fluconazole (400 mg/day) was administered as prophylaxis. On the 16th day of admission, the patient developed fever, hypotension, and demonstrated decreased mentality and was thus moved to the intensive care unit. Chest X-ray and computed tomography scans conducted on the same day showed multifocal, irregular, peribronchial consolidations and a nodular opacity, suggesting multifocal pneumonia (Fig. 1A, 1B). Antifungal therapy (liposomal amphotericin B, 150 mg/day [3 mg/kg]) was initiated on day 18 [6]. On day 20, molecular tests for respiratory viruses including severe acute respiratory syndrome coronavirus 2, Mycobacterium tuberculosis, and Pneumocystis jiroveci were negative. Streptococcus pneumoniae and Legionella urinary antigen and Mycoplasma pneumoniae and Chlamydia pneumoniae serum IgM tests were negative. On day 20, from two serial sputum cultures, a fast-growing cotton-like colony filling a Sabouraud dextrose agar plate was observed after 48 hrs of incubation at 30°C (Fig. 1C). Microscopic examination using lactophenol cotton blue revealed non-septate hyphae and pyriform sporangium with marked conical apophysis, suggesting Lichtheimia spp. (Fig. 1D, 1E).
The amphotericin B dosage was increased to 300 mg/day (6 mg/kg) on day 21 according to global mucormycosis management guidelines [6].
The minimal inhibitory concentrations (MIC) (minimal effective concentration, only for echinocandins) were determined using Sensititre YeastOne YO10 (TREK Diagnostic Systems, Cleveland, OH, USA), following CLSI M38-ED3 (Table 1) [7]. Because L. ornata is included in neither Vitek MS (bioMérieux, Marcy L’Étoile, France) nor MicroIDSys (ASTA, Suwon, Korea), these tests yielded “no identification” results. Therefore, we sequenced the D1–D2 and internal transcribed spacer (ITS) 28S rRNA gene regions as per CLSI MM18-ED2 [8]. The D1–D2 region showed 100% sequence identity with L. ornata (MG772618.1), but ITS region showed similar identity for L. corymbifera, L. ramosa, and L. ornata.
L. ornata was again isolated from sputum on day 28 but not on days 46 and 55. On day 50, when the patient showed improvement, antifungal therapy was switched to oral isavuconazole (200 mg/day). Chemotherapy was completed, and a follow-up bone marrow biopsy showed no evidence of leukemia.
Alastruey-Izquierdo et al. [9] performed antifungal susceptibility testing of Lichtheimia spp. using the EUCAST standard methodology [10] and reported that amphotericin B was the most active drug, with a 24 hr MIC of 0.015–0.5 mg/L (Table 1). In our case, despite the elevated 24 hr MIC (2 mg/L) for amphotericin B, the patient was successfully treated with it. This disparity in susceptibility test results may be attributed to methodological differences. Further studies using a commercial kit such as YeastOne and broth microdilution are needed.
Although fungal rRNA D1–D2 region sequencing identified L. ornata, considering that sequencing is time-consuming and expensive, clinical microbiologists should be able to identify Lichtheimia spp. by microscopic examination and report the results to the clinicians for appropriate, early treatment.
Notes
References
1. Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. 2011; Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 17:1859–67. DOI: 10.1111/j.1469-0691.2010.03456.x. PMID: 21199154.

2. Alvarez E, Sutton DA, Cano J, Fothergill AW, Stchigel A, Rinaldi MG, et al. 2009; Spectrum of zygomycete species identified in clinically significant specimens in the United States. J Clin Microbiol. 47:1650–6. DOI: 10.1128/JCM.00036-09. PMID: 19386856. PMCID: PMC2691065.

3. Schwartze VU, Hoffmann K, Nyilasi I, Papp T, Vagvolgyi C, de Hoog S, et al. 2012; Lichtheimia species exhibit differences in virulence potential. PLoS One. 7:e40908. DOI: 10.1371/journal.pone.0040908. PMID: 22911715. PMCID: PMC3401187. PMID: 076d85624f3d47cea894630880f1a61c.
4. Pan J, Tsui C, Li M, Xiao K, de Hoog GS, Verweij PE, et al. 2020; First case of rhinocerebral mucormycosis caused by Lichtheimia ornata, with a review of Lichtheimia infections. Mycopathologia. 185:555–67. DOI: 10.1007/s11046-020-00451-y. PMID: 32388712.

5. Chowdhary A, Gupta N, Wurster S, Kumar R, Mohabir JT, Tatavarthy S, et al. 2023; Multimodal analysis of the COVID-19-associated mucormycosis outbreak in Delhi, India indicates the convergence of clinical and environmental risk factors. Mycoses. 66:515–26. DOI: 10.1111/myc.13578. PMID: 36790120.

6. Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, et al. 2017; ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 102:433–44. DOI: 10.3324/haematol.2016.152900. PMID: 28011902. PMCID: PMC5394968.

7. CLSI. 2017. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 3rd ed. M38-ED3. Clinical and Laboratory Standards Institute;Wayne, PA: DOI: 10.1201/9781420014495-15.
8. CLSI. 2018. Interpretive criteria for identification of bacteria and fungi by targeted DNA sequencing. 2nd ed. MM18-ED2. Clinical and Laboratory Standards Institute;Wayne, PA:
9. Alastruey-Izquierdo A, Cuesta I, Walther G, Cuenca-Estrella M, Rodriguez-Tudela JL. 2010; Antifungal susceptibility profile of human-pathogenic species of Lichtheimia. Antimicrob Agents Chemother. 54:3058–60. DOI: 10.1128/AAC.01270-09. PMID: 20421405. PMCID: PMC2897292.

10. Subcommittee on antifungal susceptibility testing of the ESCMID. 2008; EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect. 14:982–4. DOI: 10.1111/j.1469-0691.2008.02086.x. PMID: 18828858.
Fig. 1
Axial chest computed tomography images showing (A) peribronchial consolidation with air-bronchogram in the right middle lobe and (B) peribronchial consolidation in the right lower lobe and a small ill-defined nodular opacity in the left lower lobe (indicated by an arrow). (C) Cotton-like colony filling a Sabouraud dextrose agar plate after 48 hrs of incubation at 30°C. (D, E) Direct microscopic examination revealing pyriform sporangium with conical apophysis and columella (lactophenol cotton blue stain, 1,000×).
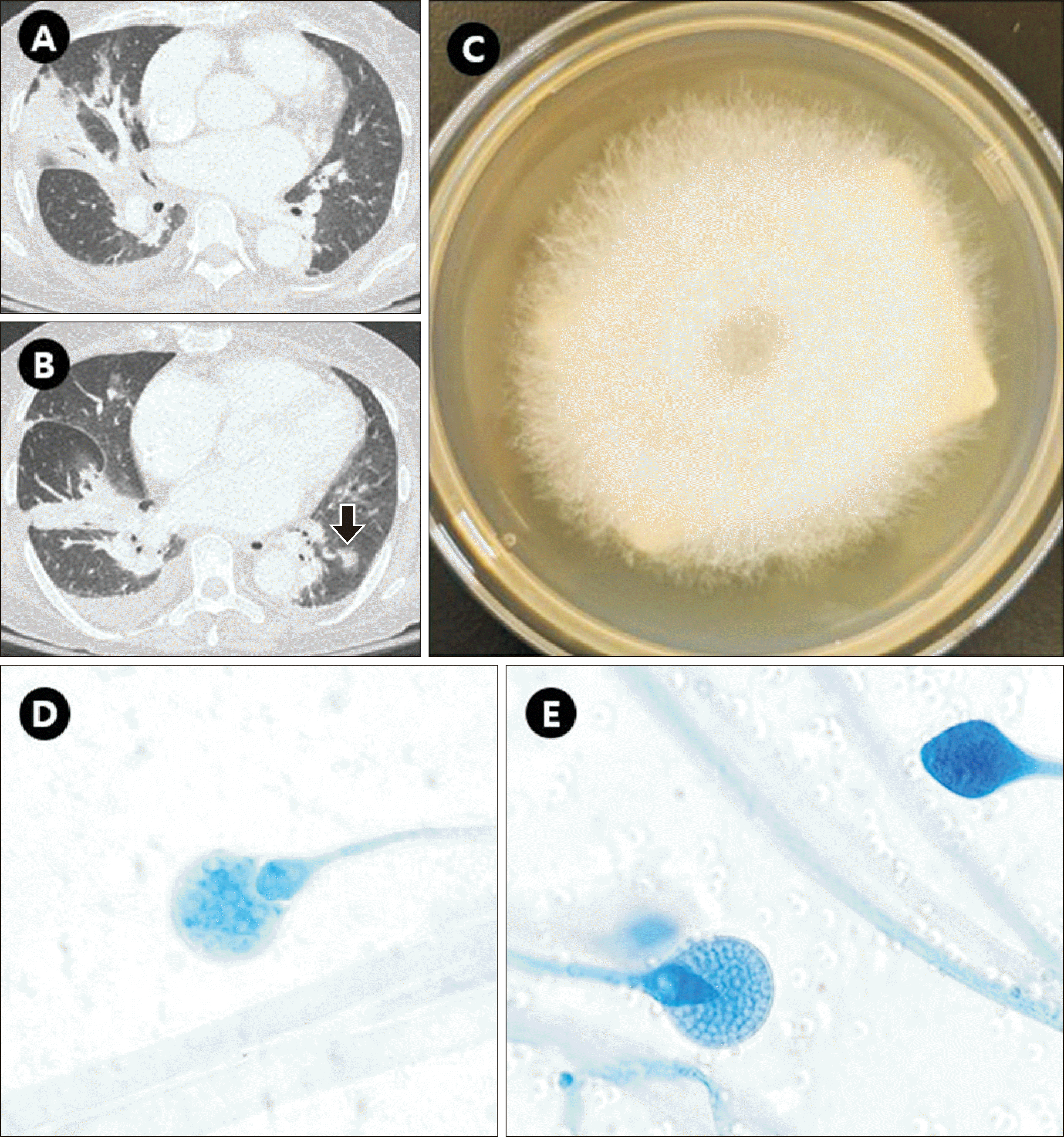
Table 1
Antifungal susceptibility of the Lichtheimia ornata isolate from this study and previously reported Lichtheimia spp. and their clinical information
| Antifungal agent | Time (hrs) assessed | MIC or MEC* (μg/mL) for: | ||
|---|---|---|---|---|
| L. ornata (this study) | L. ornata, previously reported in rhinocerebral mucormycosis [4] | L. ornata, previously reported [9] | ||
| Amphotericin B | 24/48 | 2/4 | 2/NA | 0.12/0.12 |
| Anidulafungin | 24/48 | 4/>8 | 4/32 | |
| Micafungin | 24/48 | >8/>8 | >256/NA | 32/32 |
| Caspofungin | 24/48 | >8/>8 | >16/NA | 32/32 |
| 5-flucytosine | 24/48 | >64/>64 | >64/NA | |
| Posaconazole | 24/48 | 1/1 | 2/NA | 0.25/0.25 |
| Voriconazole | 24/48 | >8/>8 | >16/NA | 16/16 |
| Itraconazole | 24/48 | 0.5/1 | 1/NA | 0.5/1 |
| Fluconazole | 24/48 | >256/>256 | >64/NA | |
| Clinical information | L. ornata (this study) | L. ornata, previously reported in rhinocerebral mucormycosis [4] | L. ornata, previously reported [9] |
|---|---|---|---|
| Age (yr)/sex | 67/F | 40/F | NA |
| Organ involvement | Lungs | Rhino-orbital-cerebral region | NA |
| Underlying condition | Acute lymphoblastic leukemia | Aplastic anemia, after allogeneic hematopoietic stem cell transplantation | NA |
| Diagnosis | Pulmonary mucormycosis | Rhinocerebral mucormycosis | NA |
| Treatment | Liposomal amphotericin B | Amphotericin B | NA |
| Outcome | Cured | Died | NA |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download