Abstract
Rh hemolytic disease of the fetus and newborn is a potential risk for D-negative mothers who produce anti-D during pregnancy, which can lead to morbidity and mortality in subsequent pregnancies. To prevent this hemolytic disease, Rho(D) immune globulin (RhIG) is generally administered to D-negative mothers without anti-D at 28 weeks of gestation and shortly after delivery. However, current guidelines suggest that pregnant mothers with molecularly defined weak D types 1, 2, 3, 4.0, and 4.1 do not need RhIG as they are unlikely to produce alloanti-D when exposed to fetuses with D-positive red cells. This issue and the necessity of RHD genotyping have been extensively discussed in Western countries, where these variants are relatively common. Recent evidence indicates that women with Asian-type DEL (c.1227G>A) also do not form alloanti-D when exposed to D-positive red cells. We report that mothers with molecularly defined Asian-type DEL, similar to those with weak D types 1, 2, 3, 4.0, and 4.1, do not require RhIG before and after delivery. Collectively, this review could pave the way for the revision of international guidelines to include the selective use of RhIG based on specific genotypes, particularly in women with the Asian-type DEL.
Hemolytic disease of the fetus and newborn (HDFN) attributed to alloanti-D can be effectively prevented when the mother receives an injection of Rho(D) immune globulin (RhIG). Therefore, RhIG is a standard management for pregnant D-negative mothers carrying D-positive fetuses or a fetus with an undetermined D type to prevent Rh incompatibility in future pregnancies [1]. However, in mothers with certain RhD variants, RhIG injections are not always necessary. RhD variants showing serologically weak D phenotypes appear as weak agglutination against anti-D reagents, whereas DEL variants serologically appear as D-negative against anti-D reagents [2, 3]. The administration of RhIG injections to pregnant women with these variants has been a topic of debate. The current consensus of the Association for the Advancement of Blood and Biotherapies and the College of American Pathologists is that RhIG immunoprophylaxis is not necessary for mothers with molecularly defined weak D types 1, 2, or 3 because they are not at risk of developing alloanti-D when sensitized with D-positive red blood cells [4]. In 2020, weak D types 4.0 and 4.1 were included in the group of molecularly defined weak D variants that do not require RhIG [5].
Weak D types 1, 2, and 3, which are the most common variants among Caucasians, and weak D types 4.0 and 4.1, which are occasionally observed in African Americans, account for approximately 80% of all weak D phenotypes in the United States [4, 5]. However, RhIG immunoprophylaxis guidelines for Asian-type DEL (c.1227G>A; RHD*DEL1), which is found in 10%–30% of serological D-negative individuals in East and Southeast Asia, have not been established [6-9]. Recent evidence suggests that pregnant mothers with Asian-type DEL do not produce alloanti-D when sensitized with D-positive fetal red cells; retrospective monitoring of 1,032 pregnant mothers with Asian-type DEL in China showed that none of them developed alloanti-D, whereas none of the 127 serologically D-negative pregnant mothers who developed alloanti-D carried Asian-type DEL [6]. Therefore, RhIG immunoprophylaxis does not appear to be necessary for mothers with Asian-type DEL, and RHD genotyping is crucial for determining whether RhIG administration is required. Asian-type DEL red cells display a complete repertoire of D antigen epitopes [10]. Alloanti-D development in transfusion recipients who received red cells from Asian-type DEL individuals has been reported in many countries [9, 11-16]. There is only one report of anti-D immunization in a multiparous Japanese woman with Asian-type DEL who received D-positive red cells; however, this does not undermine the claim that individuals with Asian-type DEL are not at risk of developing alloanti-D, as the HLA-DRB1 and -DQB1 alleles she carried are associated with anti-D alloimmunization [17].
Korean transfusion guidelines state that D-positive blood transfusion is possible in patients with molecularly defined Asian-type DEL (c.1227G>A) [18]. However, no such guidelines exist for RhIG immunoprophylaxis in mothers with Asian-type DEL. In this review, based on a comparison of the serological and molecular characteristics of Asian-type DEL with those of weak D types 1, 2, 3, 4.0, and 4.1, we suggest that RhIG immunoprophylaxis is not necessary for Asian-type DEL mothers. We also emphasize the importance of RHD genotyping.
D-negative mothers carrying D-positive fetuses may produce anti-D following fetomaternal hemorrhage at birth [19]. Because transplacental hemorrhage sufficiently large to induce sensitization generally does not occur until the third semester, routine antenatal anti-D prophylaxis is generally not administered until 28 weeks of gestation [20]. RhIG is administered as a preventive measure before and after delivery. A dose of 300 μg is injected intramuscularly (IM) at 28 weeks to prevent sensitization before delivery. An additional 300 μg IM RhIG is administered within 72 hrs after delivery. Typically, 300 μg RhIG can neutralize 15 mL of D-positive red cells [20]. When RhIG is introduced into the maternal bloodstream, anti-D antibodies attach to the D-positive red cells of the fetus. This prevents the fetal immune system from generating anti-D antibodies, and this process is believed to occur through the binding of the Fc receptor of the antibody. With routine administration of RhIG effectively protecting against rhesus alloimmunization, deaths from hemolytic disease have decreased substantially [21].
D variants can be classified as partial D, weak D, and DEL. Partial D antigens lack one or more D epitopes, whereas weak D antigens have all the D epitopes [22-26]. DEL variants refer to very weak D antigens that appear D-negative in routine serological tests but are detected when using the adsorption-elution method [2]. The DEL phenotypes have an exceedingly small number of D antigen sites per red cell. A single D-positive red cell has 9,900–33,300 D antigen sites, weak D phenotypes have 1,000–4,000 D antigen sites per red cell, and a DEL red cell has less than 22 antigen sites [23]. As accurately differentiating these types using serological methods is challenging, RHD genotyping is necessary.
Alloanti-D is produced in partial D pregnant mothers when stimulated by D-positive red cells via transfusion or pregnancy [25]. Therefore, RhIG administration to partial D mothers is recommended [27, 28]. However, pregnant mothers with molecularly defined weak D 1, 2, 3, 4.0, and 4.1 do not require RhIG. Notably, in many European countries and the United States, in cases where the genotype of the pregnant mother is determined as weak D types 1, 2, 3, 4.0, or 4.1, RhIG is not administered [5, 29-32]. Those with other weak D subtypes require RhIG. Pregnant mothers with Asian-type DEL (c.1227G>A) do not require RhIG, but those with other DEL types (i.e., IVS3+1G>A or exon 8 deletion) do.
In summary, in transfusion and RhIG immunoprophylaxis, molecularly defined D variants can be classified into two groups. The first group, which includes weak D types 1, 2, 3, 4.0, and 4.1 and Asian-type DEL, does not require RhIG and can be managed as D-positive. In contrast, the second group, comprising partial D variants along with other weak D types, including weak D type 15, which is a common D variant phenotype in East Asians [33-35], as well as other DEL types (i.e., IVS3+1G>A, exon 8 deletion), requires RhIG and should be considered and managed as D-negative individuals. Weak D type 15 should be considered D-positive, similar to weak D types 1, 2, 3, 4.0, and 4.1, as there is no evidence of alloanti-D formation [36]. However, given the amino acid change being located in the transmembrane region and the evidence of epitope loss [35, 37], alloanti-D formation is theoretically possible. Therefore, until more clinical evidence is accumulated, managing this as a D-negative type is advisable.
Weak D types 1, 2, 3, 4.0, and 4.1 and Asian-type DEL have commonalities and differences. While these two groups of variants share equivalent clinical importance concerning transfusion and RhIG administration for expectant mothers and women potentially entering pregnancy, they differ in occurrence among ethnic groups and in their serological and molecular attributes. D-negative blood type occurs in approximately 15% of European Americans, 5%–8% of Africans, and <1% of East Asians [38]. Weak D types 1, 2, and 3 account for most D variants in European Americans (>95% of serologic weak D phenotypes are attributed to these types), and weak D types 4.0 and 4.1 are common in African descendants [31].
DEL is rare in European Americans and absent in Africans [2, 39]. Most DEL variants have been identified in Japanese, Korean, Chinese, Taiwanese, and Thai [9, 40, 41], where Asian-type DEL (c.1227G>A) is the most prevalent genotype. Among 0.5% of D-negative Japanese, 0.24%-0.50% of D-negative Han Chinese, and 0.15% of D-negative Koreans, 28%, 15.4%–32.2%, and 17% have the c.1227G>A allele, respectively [42-46]. Among D-negative Thai blood donors, 15.0%–15.6% have the c.1227G>A allele [40, 41]. In DEL Koreans, RHD genotyping revealed that 94.7% had c.1227G>A, and a few had the c.1222T>C variant [44, 47]. Characteristics of weak D types 1, 2, 3, 4.0, and 4.1 and Asian-type DEL are compared in Table 1.
RHD genotyping is required to identify weak D types 1, 2, 3, 4.0, 4.1, and Asian-type DEL to avoid inappropriate RhIG administration to pregnant women (Fig. 1). The testing algorithm and RhIG and transfusion guidelines for East Asians and non-East Asians are illustrated in Fig. 1A and 1B, respectively. In the East-Asian population, weak D types 1, 2, 3, 4.0, and 4.1 are extremely rare, whereas Asian-type DEL is relatively common. Therefore, RHD genotyping is recommended for the detection of Asian-type DEL (c.1227G>A) but not for weak D types 1, 2, 3, 4.0, and 4.1 in serological weak D phenotypes [33]. Unlike for Asian-type DEL, the evidence regarding the c.1222T>C variant remains insufficient. Therefore, distinguishing c.1227G>A and c.1222T>C holds significant clinical importance. Several low-cost and effective diagnostic strategies to detect DEL (c.1227G>A) have been introduced. Studies have reported an association between the DEL phenotype and the C antigen, which was confirmed in subsequent research involving Japanese donors and D-negative European individuals [3, 48-50]. It was concluded that the DEL phenotype is associated with the Ce or cE haplotype. In regions where molecular typing of DEL is not available, some laboratories have utilized Rh CcEe phenotyping to determine the indications for RHD genotyping. Seo, et al. [47] suggested that all D-negative samples with C–E–c+e+ (ce phenotype) are D-negative owing to the complete deletion of the RHD gene, and the phenotype has 100% positive predictive value for detecting D-negative cases. All 58 samples with the C–E–c+e+ phenotype in their study showed complete RHD deletion. Based on this finding, the authors concluded that in cases where the ce phenotype is present, RHD genotyping is not necessary for approximately half of the D-negative cases [47]. Therefore, RHD genotyping is only necessary to detect DEL (c.1227G>A) in serologically D-negative mothers, except those with the ce phenotype in East-Asian populations.
In a retrospective review of 104 D-negative women with anti-D alloantibodies, none expressed the DEL variant, whereas when 199 D-negative women were prospectively investigated for RhIG, 44 (22.1%) expressed the DEL variant [51]. In a recent study, full-length RHD transcript was detected in Asian-type DEL erythroblasts that expressed the complete repertoire of D epitopes, like D-positive red cells do [6]. Pregnant women with Asian-type DEL have been clinically perceived as D-negative and, therefore, have been administered routine prophylactic RhIG. Currently, RhIG administration guidelines for serologically D-negative mothers rely on those based on European-American ethnicities. As many D-negative East Asians have Asian-type DEL, a definite guideline stating that RhIG administration is not necessary for mothers with molecularly defined Asian-type DEL must be implemented.
There have been concerns regarding the shortage of RhIG because the immunoglobulins are obtained from pooled plasma donated by volunteer D-negative individuals [52, 53]. Nevertheless, current guidelines recommend RhIG administration to all D-negative non-sensitized mothers in the third trimester and within 72 hrs of delivery or in case of other sensitizing events [1]. The omission of RhIG administration in mothers with Asian-type DEL or weak D types 1, 2, 3, 4.0, and 4.1 should be strongly considered in light of the possibility of a future immunoglobulin shortage. In addition, RhIG can cause side effects because of bloodborne pathogens from volunteer donors [54].
Multiple lines of evidence show that, similar to individuals with weak D types 1, 2, 3, 4.0, and 4.1, individuals with Asian-type DEL do not produce alloanti-D when exposed to D-positive red cells following pregnancy or transfusion. Therefore, we recommend the omission of RhIG administration for mothers with molecularly defined Asian-type DEL. This can be facilitated by a routine diagnosis of Asian-type DEL using RHD genotyping. This review significantly contributes to optimizing prenatal care in diverse populations and highlights the importance of tailored medical approaches based on genetic variations.
Notes
References
1. Qureshi H, Massey E, Kirwan D, Davies T, Robson S, White J, et al. 2014; BCSH guideline for the use of anti-D immunoglobulin for the prevention of haemolytic disease of the fetus and newborn. Transfus Med. 24:8–20. DOI: 10.1111/tme.12091. PMID: 25121158.

2. Sandler SG, Chen LN, Flegel WA. 2017; Serological weak D phenotypes: a review and guidance for interpreting the RhD blood type using the RHD genotype. Br J Haematol. 179:10–9. DOI: 10.1111/bjh.14757. PMID: 28508413. PMCID: PMC5612847.

3. Kwon DH, Sandler SG, Flegel WA. 2017; DEL phenotype. Immunohematology. 33:125–32. DOI: 10.21307/immunohematology-2019-019. PMID: 29043831. PMCID: PMC5676463.

4. Sandler SG, Flegel WA, Westhoff CM, Denomme GA, Delaney M, Keller MA, et al. 2015; It's time to phase in RHD genotyping for patients with a serologic weak D phenotype. College of American Pathologists Transfusion Medicine Resource Committee Work Group. Transfusion. 55:680–9. DOI: 10.1111/trf.12941. PMID: 25438646. PMCID: PMC4357540.

5. Flegel WA, Denomme GA, Queenan JT, Johnson ST, Keller MA, Westhoff CM, et al. 2020; It's time to phase out "serologic weak D phenotype" and resolve D types with RHD genotyping including weak D type 4. Transfusion. 60:855–9. DOI: 10.1111/trf.15741. PMID: 32163599. PMCID: PMC9121350.

6. Ji Y, Luo Y, Wen J, Sun Y, Jia S, Ou C, et al. 2023; Patients with Asian-type DEL can safely be transfused with RhD-positive blood. Blood. 141:2141–50. DOI: 10.1182/blood.2022018152. PMID: 36638337. PMCID: PMC10273079.
7. Choi S, Chun S, Seo JY, Yang JH, Cho D. 2019; Planned transfusion of D-positive blood components in an Asia type DEL patient: proposed modification of the Korean national guidelines for blood transfusion. Ann Lab Med. 39:102–4. DOI: 10.3343/alm.2019.39.1.102. PMID: 30215239. PMCID: PMC6143459.

8. Hess JR. 2023; Safe transfusion in Asian-type DEL. Blood. 141:2044–6. DOI: 10.1182/blood.2023019646. PMID: 37103952.

9. Thongbut J, Raud L, Férec C, Promwong C, Nuchnoi P, Fichou Y. 2020; Comprehensive molecular analysis of serologically D-negative and weak/partial D phenotype in Thai Blood donors. Transfus Med Hemother. 47:54–60. DOI: 10.1159/000499087. PMID: 32110194. PMCID: PMC7036540.

10. Körmöczi GF, Gassner C, Shao CP, Uchikawa M, Legler TJ. 2005; A comprehensive analysis of DEL types: partial DEL individuals are prone to anti-D alloimmunization. Transfusion. 45:1561–7. DOI: 10.1111/j.1537-2995.2005.00584.x. PMID: 16181205.

11. Wagner T, Körmöczi GF, Buchta C, Vadon M, Lanzer G, Mayr WR, et al. 2005; Anti-D immunization by DEL red blood cells. Transfusion. 45:520–6. DOI: 10.1111/j.0041-1132.2005.04256.x. PMID: 15819672.

12. Yasuda H, Ohto H, Sakuma S, Ishikawa Y. 2005; Secondary anti-D immunization by Del red blood cells. Transfusion. 45:1581–4. DOI: 10.1111/j.1537-2995.2005.00579.x. PMID: 16181208.

13. Kim KH, Kim KE, Woo KS, Han JY, Kim JM, Park KU. 2009; Primary anti-D immunization by DEL red blood cells. Korean J Lab Med. 29:361–5. DOI: 10.3343/kjlm.2009.29.4.361. PMID: 19726900.

14. Yang HS, Lee MY, Park TS, Cho SY, Lee HJ, Lim G, et al. 2015; Primary anti-D alloimmunization induced by "Asian type" RHD (c.1227G>A) DEL red cell transfusion. Ann Lab Med. 35:554–6. DOI: 10.3343/alm.2015.35.5.554. PMID: 26206698. PMCID: PMC4510514.

15. Shao CP, Wang BY, Ye SH, Zhang WL, Xu H, Zhuang NB, et al. 2012; DEL RBC transfusion should be avoided in particular blood recipient in East Asia due to allosensitization and ineffectiveness. J Zhejiang Univ Sci B. 13:913–8. DOI: 10.1631/jzus.B1100348. PMID: 23125084. PMCID: PMC3494030.

16. Thongbut J, Bénech C, Phiri N, Suwanwootichai P, Thongpao C, Bejrachandra S, et al. 2023; Anti-D alloimmunization by Asia type DEL red blood cell units in a D-negative Thai patient. Transfus Apher Sci. 62:103837. DOI: 10.1016/j.transci.2023.103837. PMID: 37872073.

17. Ohto H, Ito S, Srivastava K, Ogiyama Y, Uchikawa M, Nollet KE, et al. 2023; Asian-type DEL (RHD*DEL1) with an allo-anti-D: a paradoxical observation in a healthy multiparous woman. Transfusion. 63:1601–11. DOI: 10.1111/trf.17465. PMID: 37465939.

18. KCDC. 2022. National transfusion guideline. Korea Centers for Disease Control and Prevention;Seoul, Republic of Korea: DOI: 10.17945/kjbt.2016.27.2.155.
19. Chown B. 1954; Anaemia from bleeding of the fetus into the mother's circulation. Lancet. 266:1213–5. DOI: 10.1016/S0140-6736(54)92446-0. PMID: 13164357.
20. 2017; Practice Bulletin No. 181: Prevention of Rh D alloimmunization. Obstet Gynecol. 130:e57–70. DOI: 10.1097/AOG.0000000000002232. PMID: 28742673.
21. Ware RE, Zimmerman SA. 1998; Anti-D: mechanisms of action. Semin Hematol. 35(S1):14–22.
22. Avent ND, Reid ME. 2000; The Rh blood group system: a review. Blood. 95:375–87. DOI: 10.1182/blood.V95.2.375. PMID: 10627438.

23. Wagner FF, Frohmajer A, Ladewig B, Eicher NI, Lonicer CB, Müller TH, et al. 2000; Weak D alleles express distinct phenotypes. Blood. 95:2699–708. DOI: 10.1182/blood.V95.8.2699. PMID: 10753853.

24. Daniels G. 2013; Variants of RhD-current testing and clinical consequences. Br J Haematol. 161:461–70. DOI: 10.1111/bjh.12275. PMID: 23432139.

25. Westhoff CM. 2007; The structure and function of the Rh antigen complex. Semin Hematol. 44:42–50. DOI: 10.1053/j.seminhematol.2006.09.010. PMID: 17198846. PMCID: PMC1831834.

26. Lee GH, Kim H, Hur M, So KA, Shin DW, Hong YJ, et al. 2023; RHD*DNT (RHD*38) showing D-positive reactivity on rhesus D typing and forming anti-D antibody. Ann Lab Med. 43:524–7. DOI: 10.3343/alm.2023.43.5.524. PMID: 37080757. PMCID: PMC10151267.

27. Tippett P, Lomas-Francis C, Wallace M. 1996; The Rh antigen D: partial D antigens and associated low incidence antigens. Vox Sang. 70:123–31. DOI: 10.1111/j.1423-0410.1996.tb01309.x. PMID: 8740002.

28. Lubenko A, Contreras M, Habash J. 1989; Should anti-Rh immunoglobulin be given D variant women? Br J Haematol. 72:429–33. DOI: 10.1111/j.1365-2141.1989.tb07727.x. PMID: 2504271.

29. Levitt J, editor. 2014. Standards for blood banks and transfusion services. 29th ed. AABB Press;Bethesda, MD:
30. Westhoff CM, Nance S, Lomas-Francis C, Keller M, Chou ST. 2019; Experience with RHD*weak D type 4.0 in the USA. Blood Transfus. 17:91–3.
31. Flegel WA, Peyrard T, Chiaroni J, Tournamille C, Jamet D, Pirenne F. 2019; A proposal for a rational transfusion strategy in patients of European and North African descent with weak D type 4.0 and 4.1 phenotypes. Blood Transfus. 17:89–90.
32. Flegel WA, Bodnar M, Clarke G, Hannon J, Lieberman L. 2021; What constitutes the most cautious approach for a pregnant person with weak D type 4.0? CMAJ. 193:E916. DOI: 10.1503/cmaj.78986. PMID: 34860699. PMCID: PMC8248459.

33. Chung YN, Kim TY, Yu H, Bae JC, Cho D. 2021; Molecular basis of serological weak D phenotypes and RhD typing discrepancies identified in the Korean population. Blood Transfus. 19:327–34.
34. Takeuchi-Baba C, Ito S, Kinjo R, Miyagi H, Yasuda H, Ogasawara K, et al. 2019; Production of RBC autoantibody mimicking anti-D specificity following transfusion in a patient with weak D Type 15. Transfusion. 59:1190–5. DOI: 10.1111/trf.15207. PMID: 30784074.

35. Wen J, Jia S, Wang Z, Chen J, Liang Q, Wei L, et al. 2023; Molecular and serological analysis of the D variant in the Chinese population and identification of seven novel RHD alleles. Transfusion. 63:402–14. DOI: 10.1111/trf.17186. PMID: 36382965.

36. Flegel WA. 2021; Proceed with care: the "uncommon" serologic weak D phenotypes. Blood Transfus. 19:272–6.
37. Wagner FF, Gassner C, Müller TH, Schönitzer D, Schunter F, Flegel WA. 1999; Molecular basis of weak D phenotypes. Blood. 93:385–93. DOI: 10.1182/blood.V93.1.385. PMID: 9864185.

38. Daniels G. 2013. Human blood groups. 3d ed. Wiley-Blackwell;West Sussex, UK: DOI: 10.1002/9781118493595.
39. Flegel WA. 2011; Molecular genetics and clinical applications for RH. Transfus Apher Sci. 44:81–91. DOI: 10.1016/j.transci.2010.12.013. PMID: 21277262. PMCID: PMC3042511.

40. Thongbut J, Laengsri V, Raud L, Promwong C, I-Na-Ayudhya C, Férec C, et al. 2021; Nation-wide investigation of RHD variants in Thai blood donors: impact for molecular diagnostics. Transfusion. 61:931–8. DOI: 10.1111/trf.16242. PMID: 33377204.

41. Nuchnoi P, Thongbut J, Bénech C, Kupatawintu P, Chaiwanichsiri D, Férec C, et al. 2023; Serologically D-negative blood donors in Thailand: molecular variants and diagnostic strategy. Blood Transfus. 21:209–17. DOI: 10.2450/2022.0160-22. PMID: 36346882. PMCID: PMC10159805.
42. Yin Q, Flegel WA. 2021; DEL in China: the D antigen among serologic RhD-negative individuals. J Transl Med. 19:439. DOI: 10.1186/s12967-021-03116-6. PMID: 34670559. PMCID: PMC8527646. PMID: c813824e35614bdb82995b849845a27c.

43. Shao CP, Maas JH, Su YQ, Kohler M, Legler TJ. 2002; Molecular background of Rh D-positive, D-negative, D(el) and weak D phenotypes in Chinese. Vox Sang. 83:156–61. DOI: 10.1046/j.1423-0410.2002.00192.x. PMID: 12201845.
44. Kim JY, Kim SY, Kim CA, Yon GS, Park SS. 2005; Molecular characterization of D- Korean persons: development of a diagnostic strategy. Transfusion. 45:345–52. DOI: 10.1111/j.1537-2995.2005.04311.x. PMID: 15752151.

45. Okubo Y, Seno T, Yamano H, Yamaguchi H, Lomas C, Tippett P. 1991; Partial D antigens disclosed by a monoclonal anti-D in Japanese blood donors. Transfusion. 31:782. DOI: 10.1046/j.1537-2995.1991.31892023512.x. PMID: 1926326.

46. Luettringhaus TA, Cho D, Ryang DW, Flegel WA. 2006; An easy RHD genotyping strategy for D- East Asian persons applied to Korean blood donors. Transfusion. 46:2128–37. DOI: 10.1111/j.1537-2995.2006.01042.x. PMID: 17176325.

47. Seo MH, Won EJ, Hong YJ, Chun S, Kwon JR, Choi YS, et al. 2016; An effective diagnostic strategy for accurate detection of RhD variants including Asian DEL type in apparently RhD-negative blood donors in Korea. Vox Sang. 111:425–30. DOI: 10.1111/vox.12450. PMID: 27864976.
48. Okubo Y, Yamaguchi H, Tomita T, Nagao N. 1984; A D variant, Del? Transfusion. 24:542. DOI: 10.1046/j.1537-2995.1984.24685066827.x. PMID: 6438843.
49. Okuda H, Kawano M, Iwamoto S, Tanaka M, Seno T, Okubo Y, et al. 1997; The RHD gene is highly detectable in RhD-negative Japanese donors. J Clin Invest. 100:373–9. DOI: 10.1172/JCI119543. PMID: 9218514. PMCID: PMC508200.
50. Wagner FF, Flegel WA. 2014; The rhesus site. Transfus Med Hemother. 41:357–63. DOI: 10.1159/000366176. PMID: 25538538. PMCID: PMC4264492.

51. Shao CP. 2010; Transfusion of RhD-positive blood in "Asia type" DEL recipients. N Engl J Med. 362:472–3. DOI: 10.1056/NEJMc0909552. PMID: 20130261.

52. Beveridge HE. 1997; Dwindling supplies of anti-D. Med J Aust. 167:509–10. DOI: 10.5694/j.1326-5377.1997.tb126698.x. PMID: 9397073.

53. Robson SC, Lee D, Urbaniak S. 1998; Anti-D immunoglobulin in RhD prophylaxis. Br J Obstet Gynaecol. 105:129–34. DOI: 10.1111/j.1471-0528.1998.tb10039.x. PMID: 9501773.

54. Mark A, Foster AM, Grossman D, Prager SW, Reeves M, Velásquez CV, et al. 2019; Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation's Clinical Policies Committee. Contraception. 99:265–6. DOI: 10.1016/j.contraception.2019.02.008. PMID: 30867121.

Fig. 1
Flowchart for RhD typing. (A) Serologic and genetic testing of Asian-type DEL with determination algorithm of Rho(D) immune globulin in East Asians. (B) Serologic and genetic testing of weak D types 1, 2, 3, 4.0, and 4.1 with determination algorithm of Rho(D) immune globulin in non-East Asians.
Abbreviations: RBC, red blood cells; RhIG, Rho(D) immune globulin.
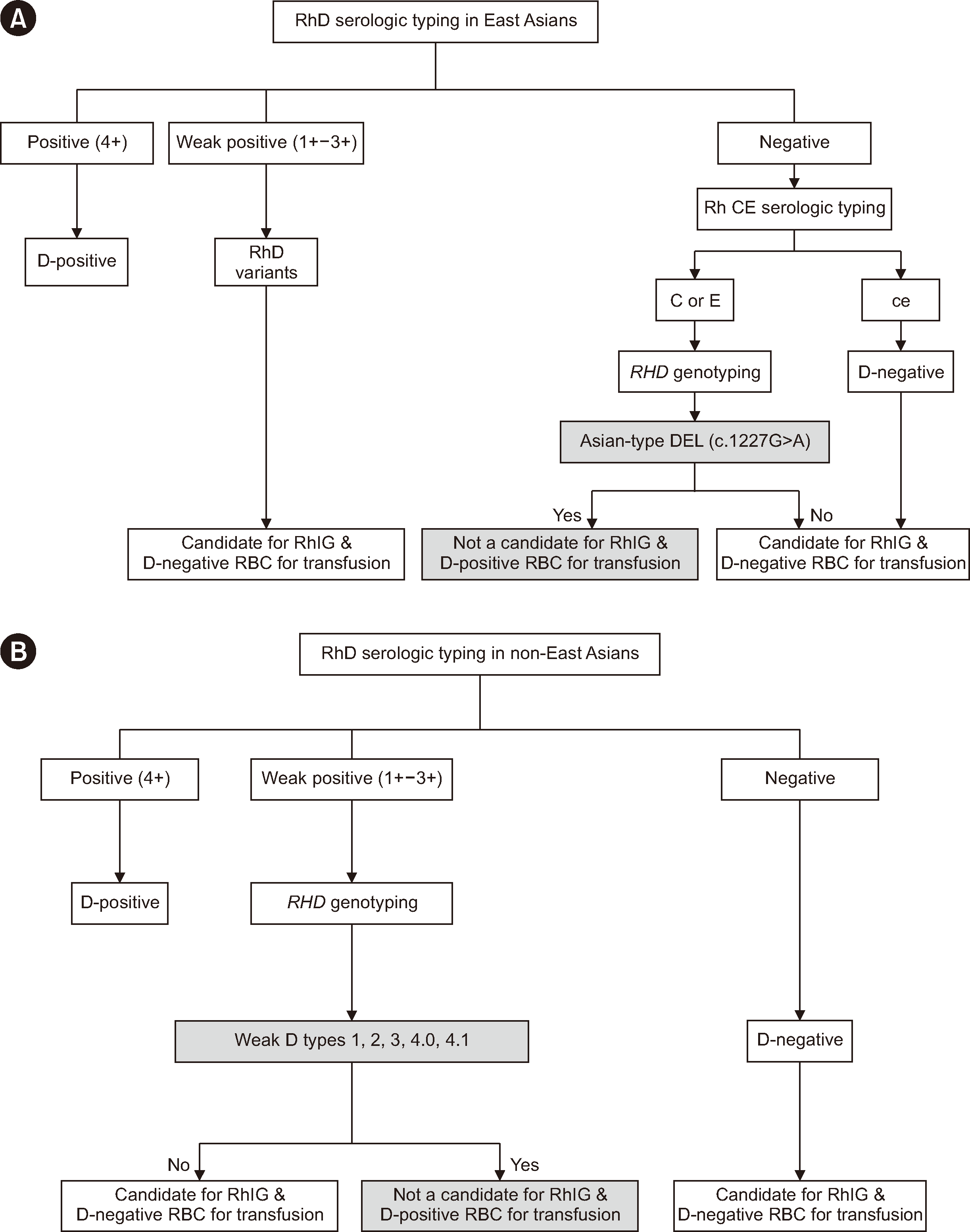
Table 1
Characteristics of individuals with weak D types 1, 2, 3, 4.0, 4.1, and Asian-type DEL and guidance for Rho(D) immune globulin administration




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download