1. World Health Organization. WHO coronavirus (COVID-19) dashboard. Updated 2023. Accessed August 22, 2023.
https://covid19.who.int/
.
2. Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020; 24(1):516. PMID:
32825837.
3. Lobo SM, Creutzfeldt CJ, Maia IS, Town JA, Amorim E, Kross EK, et al. Perceptions of critical care shortages, resource use, and provider well-being during the COVID-19 pandemic: a survey of 1,985 health care providers in Brazil. Chest. 2022; 161(6):1526–1542. PMID:
35150658.
4. Wise J. Covid-19: WHO declares end of global health emergency. BMJ. 2023; 381:1041. PMID:
37160309.
5. Telenti A, Arvin A, Corey L, Corti D, Diamond MS, García-Sastre A, et al. After the pandemic: perspectives on the future trajectory of COVID-19. Nature. 2021; 596(7873):495–504. PMID:
34237771.
6. Combes A, Schmidt M, Hodgson CL, Fan E, Ferguson ND, Fraser JF, et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020; 46(12):2464–2476. PMID:
33140180.
7. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021; 47(11):1181–1247. PMID:
34599691.
8. Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021; 67(5):485–495. PMID:
33657573.
9. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009; 374(9698):1351–1363. PMID:
19762075.
10. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018; 378(21):1965–1975. PMID:
29791822.
11. Baek MS, Lee SM, Chung CR, Cho WH, Cho YJ, Park S, et al. Improvement in the survival rates of extracorporeal membrane oxygenation-supported respiratory failure patients: a multicenter retrospective study in Korean patients. Crit Care. 2019; 23(1):1. PMID:
30606235.
12. Makhoul M, Keizman E, Carmi U, Galante O, Ilgiyaev E, Matan M, et al. Outcomes of extracorporeal membrane oxygenation (ECMO) for COVID-19 patients: a multi-institutional analysis. Vaccines (Basel). 2023; 11(1):108. PMID:
36679953.
13. Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015; 191(8):894–901. PMID:
25695688.
14. Nishikimi M, Ohshimo S, Hamaguchi J, Fujizuka K, Hagiwara Y, Anzai T, et al. High versus low positive end-expiratory pressure setting in patients receiving veno-venous extracorporeal membrane oxygenation support for severe acute respiratory distress syndrome: study protocol for the multicentre, randomised ExPress SAVER Trial. BMJ Open. 2023; 13(10):e072680.
15. Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021; 25(1):211. PMID:
34127027.
16. Ling RR, Ramanathan K, Sim JJ, Wong SN, Chen Y, Amin F, et al. Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: a systematic review and meta-analysis. Crit Care. 2022; 26(1):147. PMID:
35606884.
17. Chong WH, Saha BK, Medarov BI. Clinical characteristics between survivors and nonsurvivors of COVID-19 patients requiring extracorporeal membrane oxygenation (ECMO) support: a systematic review and meta-analysis. J Intensive Care Med. 2022; 37(3):304–318. PMID:
34636697.
18. Tran A, Fernando SM, Rochwerg B, Barbaro RP, Hodgson CL, Munshi L, et al. Prognostic factors associated with mortality among patients receiving venovenous extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Lancet Respir Med. 2023; 11(3):235–244. PMID:
36228638.
19. Chong WH, Saha BK, Medarov BI. A systematic review and meta-analysis comparing the clinical characteristics and outcomes of COVID-19 and influenza patients on ECMO. Respir Investig. 2021; 59(6):748–756.
20. Fanelli V, Giani M, Grasselli G, Mojoli F, Martucci G, Grazioli L, et al. Extracorporeal membrane oxygenation for COVID-19 and influenza H1N1 associated acute respiratory distress syndrome: a multicenter retrospective cohort study. Crit Care. 2022; 26(1):34. PMID:
35123562.
21. Kurihara C, Manerikar A, Gao CA, Watanabe S, Kandula V, Klonis A, et al. Outcomes after extracorporeal membrane oxygenation support in COVID-19 and non-COVID-19 patients. Artif Organs. 2022; 46(4):688–696. PMID:
34694655.
22. Ling RR, Ramanathan K, Poon WH, Tan CS, Brechot N, Brodie D, et al. Venoarterial extracorporeal membrane oxygenation as mechanical circulatory support in adult septic shock: a systematic review and meta-analysis with individual participant data meta-regression analysis. Crit Care. 2021; 25(1):246. PMID:
34261492.
23. Oh TK, Cho HW, Song IA. Mortality trends after extracorporeal membrane oxygenation support: a Korean nationwide cohort. Artif Organs. 2022; 46(5):850–858. PMID:
35083743.
24. Schmidt M, Langouet E, Hajage D, James SA, Chommeloux J, Bréchot N, et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals, Paris. Crit Care. 2021; 25(1):355. PMID:
34627350.
25. Riera J, Alcántara S, Bonilla C, Fortuna P, Blandino Ortiz A, Vaz A, et al. Risk factors for mortality in patients with COVID-19 needing extracorporeal respiratory support. Eur Respir J. 2022; 59(2):2102463. PMID:
34824058.
26. Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021; 9(8):851–862. PMID:
33887246.
27. Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021; 398(10307):1230–1238. PMID:
34599878.
28. Supady A, Taccone FS, Lepper PM, Ziegeler S, Staudacher DL. COVEC-Study Group. Survival after extracorporeal membrane oxygenation in severe COVID-19 ARDS: results from an international multicenter registry. Crit Care. 2021; 25(1):90. PMID:
33648538.
29. Shaefi S, Brenner SK, Gupta S, O’Gara BP, Krajewski ML, Charytan DM, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021; 47(2):208–221. PMID:
33528595.
30. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014; 189(11):1374–1382. PMID:
24693864.
31. Joshi H, Flanagan M, Subramanian R, Drouin M. Respiratory ECMO Survival Prediction (RESP) score for COVID-19 patients treated with ECMO. ASAIO J. 2022; 68(4):486–491. PMID:
35239533.
32. Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, et al. Extracorporeal Life Support Organization Coronavirus Disease 2019 Interim Guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020; 66(7):707–721. PMID:
32604322.
33. Supady A, Combes A, Barbaro RP, Camporota L, Diaz R, Fan E, et al. Respiratory indications for ECMO: focus on COVID-19. Intensive Care Med. 2022; 48(10):1326–1337. PMID:
35945343.
34. Roberts SH, Goodwin ML, Bobba CM, Al-Qudsi O, Satyapriya SV, Tripathi RS, et al. Continuous renal replacement therapy and extracorporeal membrane oxygenation: implications in the COVID-19 era. Perfusion. 2023; 38(1):18–27. PMID:
34494489.
35. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270–273. PMID:
32015507.
36. Tang L, Yin Z, Hu Y, Mei H. Controlling cytokine storm is vital in COVID-19. Front Immunol. 2020; 11:570993. PMID:
33329533.
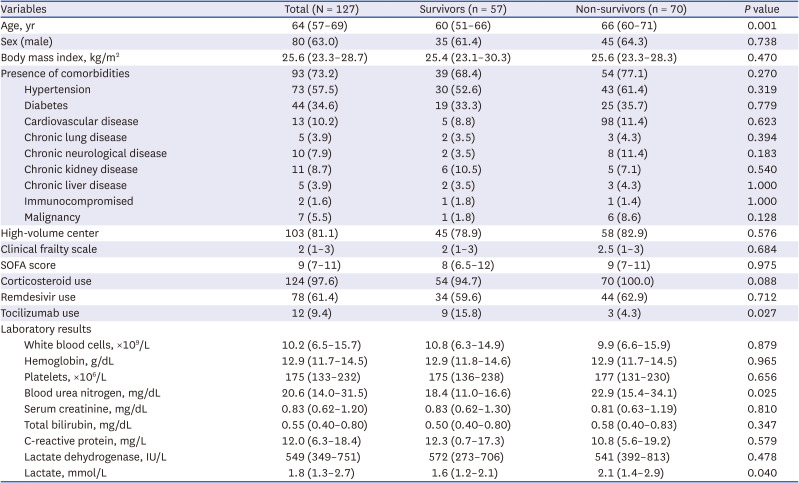
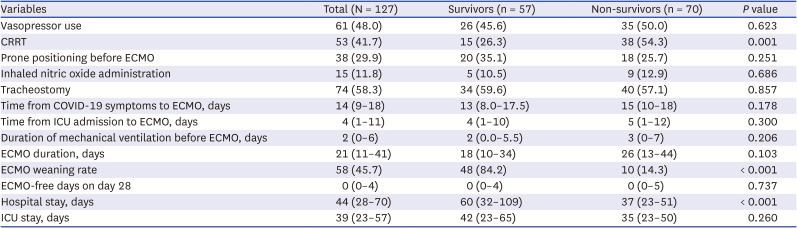
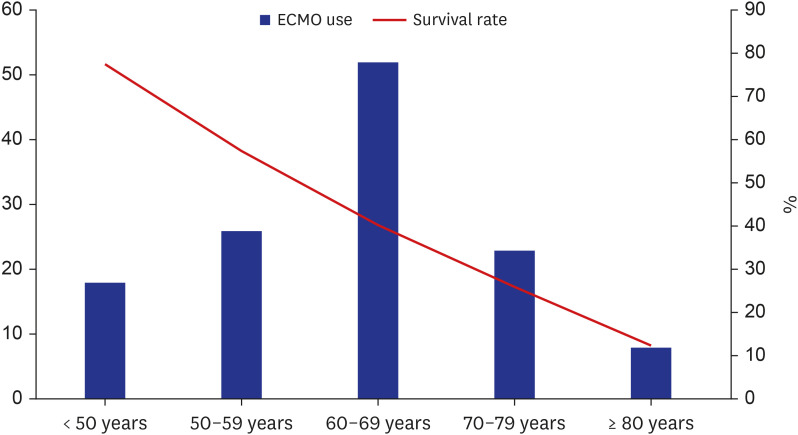
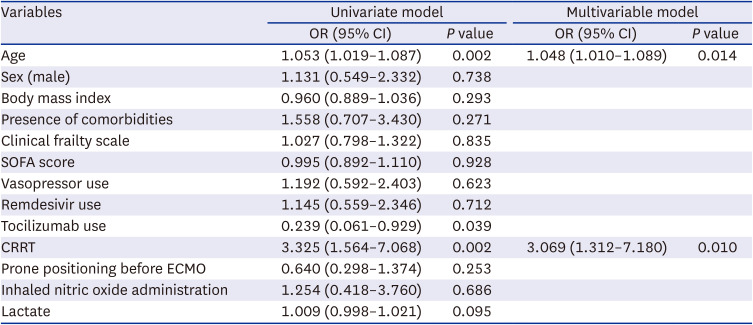




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download